Hydrogen bonding, a critical concept in organic chemistry, significantly influences the physical properties of ethanol. The University of Example State Chemistry Department’s research consistently highlights the role of hydrogen bonding in various alcoholic solutions. Spectroscopy, a powerful analytical tool, is often used to investigate these interactions. Furthermore, the understanding of van der Waals forces is crucial to comprehending the full spectrum of intermolecular forces in ethanol and how they dictate its behavior as a solvent and reactant.
Ethanol: More Than Just Fuel
Ethanol, a clear, colorless liquid with a characteristic odor, is a compound familiar to us in various guises. From its ubiquitous presence as a fuel additive in gasoline to its role as a solvent in countless industrial processes, and even as a disinfectant in healthcare, ethanol’s versatility is undeniable.
A Common Compound with Complex Origins
It’s a component of alcoholic beverages, a key ingredient in hand sanitizers, and a building block for the synthesis of numerous chemicals.
This widespread utilization often overshadows the intricate molecular interactions that dictate ethanol’s unique properties.
Unveiling the Invisible Forces
While we readily observe and exploit ethanol’s macroscopic behaviors – its flammability, its miscibility with water, its ability to dissolve other substances – the underlying forces at play remain largely unseen.
These intermolecular forces, arising from the electromagnetic interactions between ethanol molecules, are the key to understanding why ethanol behaves the way it does. They determine its boiling point, its solubility, and its interactions with other compounds.
The Macroscopic Properties of Ethanol
Delving into these forces allows us to decode the relationship between ethanol’s molecular structure and its observable characteristics, bridging the gap between the microscopic and macroscopic worlds.
The properties we take for granted, such as its relatively high boiling point compared to other organic compounds of similar molecular weight, are a direct consequence of these interactions.
Thesis Statement: A Deep Dive into Intermolecular Forces
This article embarks on a journey to explore the nature and impact of intermolecular forces on ethanol’s macroscopic properties.
By examining the specific types of forces at play, we aim to provide a comprehensive understanding of how these interactions shape the behavior of this seemingly simple, yet remarkably complex, molecule.
Delving into these forces allows us to decode the relationship between ethanol’s molecular structure and its observable characteristics, bridging the gap between the microscopic and macroscopic worlds. To fully grasp the nature of these interactions, it’s essential to first dissect the molecular architecture of ethanol itself.
Ethanol’s Molecular Architecture: A Closer Look
Ethanol, represented by the chemical formula C2H5OH, is a relatively simple molecule with profound implications for its physical and chemical properties. Its structure comprises two primary components: an ethyl group (C2H5) and a hydroxyl group (-OH). This particular arrangement is not arbitrary; it’s the key to understanding ethanol’s unique behavior.
The Ethyl Group: A Hydrocarbon Foundation
The ethyl group (C2H5) consists of two carbon atoms and five hydrogen atoms.
This portion of the ethanol molecule is hydrophobic, meaning it tends to repel water.
It is derived from ethane, a simple alkane, and provides the non-polar character to ethanol.
The Hydroxyl Group: The Functional Heart
Attached to the ethyl group is the hydroxyl group (-OH), comprised of an oxygen atom and a hydrogen atom.
The hydroxyl group is polar and hydrophilic, meaning it is attracted to water and other polar molecules.
This group is the workhorse of ethanol’s intermolecular interactions, being responsible for hydrogen bonding and influencing other forces.
The presence of this highly electronegative oxygen atom is particularly crucial.
The Significance of the Hydroxyl Group
The hydroxyl group’s presence is paramount in dictating ethanol’s behavior.
It’s the primary site for hydrogen bonding, a strong intermolecular force that significantly elevates ethanol’s boiling point compared to other organic compounds of similar molecular weight.
The hydroxyl group also contributes to ethanol’s solubility in water, allowing it to form hydrogen bonds with water molecules.
Without the hydroxyl group, ethanol would behave more like its hydrocarbon cousin, ethane, and would be a gas at room temperature with limited solubility in water.
Atomic Electronegativity: The Driving Force
The individual atoms within the ethanol molecule—carbon, hydrogen, and, most importantly, oxygen—each contribute to the overall molecular properties due to their differing electronegativities.
Electronegativity refers to an atom’s ability to attract electrons within a chemical bond.
Oxygen is significantly more electronegative than both carbon and hydrogen.
This difference in electronegativity leads to an unequal sharing of electrons in the O-H bond of the hydroxyl group, creating a partial negative charge (δ-) on the oxygen atom and a partial positive charge (δ+) on the hydrogen atom.
This charge separation is what enables hydrogen bonding and contributes to the polar nature of ethanol.
The carbon-oxygen bond also exhibits a dipole moment because of the difference in electronegativity between carbon and oxygen.
Delving into these forces allows us to decode the relationship between ethanol’s molecular structure and its observable characteristics, bridging the gap between the microscopic and macroscopic worlds. To fully grasp the nature of these interactions, it’s essential to first dissect the molecular architecture of ethanol itself.
With a firm understanding of ethanol’s structure, we can now explore the forces that govern its interactions. Among these, one reigns supreme, dictating many of ethanol’s characteristic properties.
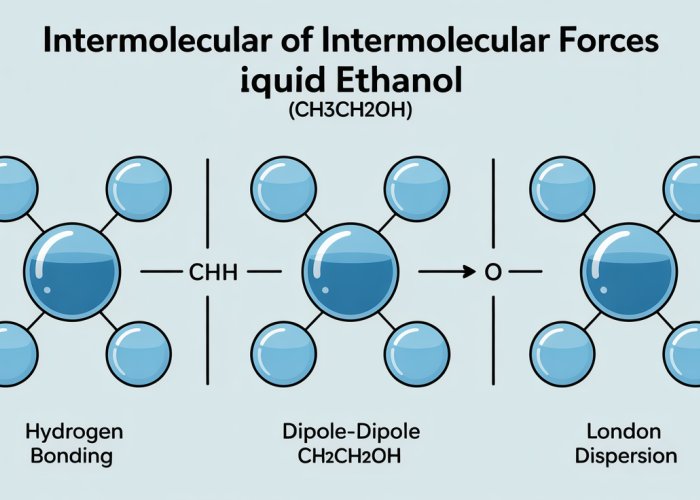
Hydrogen Bonding: The Dominant Force in Ethanol
Intermolecular forces are the unseen architects of matter, dictating how molecules interact and, consequently, how substances behave. While various such forces exist, hydrogen bonding stands out as a particularly potent one, and it plays a crucial role in shaping ethanol’s properties.
Defining Hydrogen Bonding
Hydrogen bonding is not a true chemical bond in the traditional sense, like a covalent or ionic bond. Instead, it is a strong type of intermolecular force that arises from the attraction between a partially positive hydrogen atom and a highly electronegative atom, such as oxygen, nitrogen, or fluorine.
This attraction occurs when a hydrogen atom is already covalently bonded to one of these electronegative atoms, creating a significant dipole moment. The hydrogen atom, now bearing a partial positive charge (δ+), is drawn to the lone pair of electrons on the electronegative atom of a neighboring molecule, which carries a partial negative charge (δ-).
The Hydroxyl Group’s Role in Facilitating Hydrogen Bonding
Ethanol, with its characteristic hydroxyl group (-OH), is an ideal candidate for hydrogen bonding. The oxygen atom in the hydroxyl group is highly electronegative, drawing electron density away from the hydrogen atom to which it is bonded.
This creates a significant dipole moment within the O-H bond, resulting in a partially positive hydrogen atom poised to interact with the partially negative oxygen atom of another ethanol molecule.
The hydroxyl group, therefore, acts as both a hydrogen bond donor (through its hydrogen atom) and a hydrogen bond acceptor (through its oxygen atom), facilitating extensive networks of hydrogen bonds between ethanol molecules.
The Mechanism of Hydrogen Bonding in Ethanol
The hydrogen bonding interaction in ethanol can be visualized as follows: Imagine two ethanol molecules approaching each other. As the partially positive hydrogen atom of the hydroxyl group on one ethanol molecule nears the partially negative oxygen atom of the hydroxyl group on the other, an electrostatic attraction begins to form.
This attraction is directional, meaning it is strongest when the three atoms involved (O-H···O) are aligned in a nearly linear arrangement. The strength of a typical hydrogen bond in ethanol ranges from 5 to 30 kJ/mol, significantly stronger than other intermolecular forces such as Van der Waals forces.
These hydrogen bonds are constantly forming and breaking as molecules move and collide, yet their collective effect is substantial. The hydrogen bonds create a network of interconnected molecules, influencing ethanol’s physical properties and dictating its interactions with other substances.
With hydrogen bonding so prominently influencing ethanol’s behavior, it might be easy to overlook other intermolecular forces at play. However, even though they’re generally weaker, Van der Waals forces also contribute to the overall intermolecular landscape, shaping ethanol’s properties in subtle but significant ways. These forces, arising from temporary fluctuations in electron distribution and permanent molecular dipoles, add another layer of complexity to our understanding of this versatile molecule.
Van der Waals Forces: Contributing to Ethanol’s Intermolecular Landscape
While hydrogen bonding takes center stage in dictating many of ethanol’s properties, a supporting cast of intermolecular forces, collectively known as Van der Waals forces, also plays a role. Though weaker than hydrogen bonds, these forces contribute to the overall interaction between ethanol molecules and influence certain aspects of its behavior.
Understanding Van der Waals Forces
Van der Waals forces are a group of relatively weak intermolecular forces that arise from temporary or induced dipoles in molecules. Unlike hydrogen bonds, which require specific electronegative atoms bonded to hydrogen, Van der Waals forces are universally present between all molecules.
They are distance-dependent, meaning their strength decreases rapidly with increasing separation between molecules.
Van der Waals forces are typically categorized into:
- London dispersion forces
- Dipole-dipole interactions
- Dipole-induced dipole interactions.
We’ll explore the first two, as they are the most relevant to understanding ethanol’s intermolecular interactions.
London Dispersion Forces: The Universal Attraction
London dispersion forces, also known as induced dipole-induced dipole interactions, are the weakest type of Van der Waals force.
They arise from temporary fluctuations in electron distribution within a molecule.
At any given instant, the electron cloud around an atom or molecule may become unevenly distributed, creating a temporary, instantaneous dipole. This temporary dipole can then induce a dipole in a neighboring molecule, leading to a weak, short-lived attraction.
While individually weak, London dispersion forces become more significant in molecules with larger surface areas or greater numbers of electrons.
The ethyl group (C2H5) in ethanol contributes to the overall London dispersion forces due to its size and number of electrons.
Dipole-Dipole Interactions: Leveraging Molecular Polarity
Dipole-dipole interactions occur between polar molecules, which have a permanent separation of charge due to differences in electronegativity between their constituent atoms.
Ethanol is a polar molecule due to the electronegativity difference between oxygen and hydrogen in the hydroxyl group (-OH). This creates a permanent dipole moment, with the oxygen atom bearing a partial negative charge (δ-) and the hydrogen atom bearing a partial positive charge (δ+).
The partially negative end of one ethanol molecule is attracted to the partially positive end of another, resulting in dipole-dipole interactions.
These interactions are stronger than London dispersion forces but weaker than hydrogen bonds.
They contribute to the overall attractive forces between ethanol molecules, influencing its physical properties.
Polarity’s Influence on Intermolecular Interactions
The polarity of the ethanol molecule is a key factor in determining the strength and nature of its intermolecular interactions.
The hydroxyl group (-OH) is responsible for both the hydrogen bonding and the dipole-dipole interactions observed in ethanol.
The electronegativity difference between oxygen and hydrogen creates a significant dipole moment, making ethanol a polar molecule. This polarity not only enables hydrogen bonding but also enhances dipole-dipole interactions.
The nonpolar ethyl group (C2H5) also plays a role, contributing to London dispersion forces. The interplay between these polar and nonpolar regions gives ethanol its unique properties, including its ability to dissolve in both polar and nonpolar solvents to some extent.
In summary, while hydrogen bonding dominates ethanol’s intermolecular interactions, Van der Waals forces, including London dispersion forces and dipole-dipole interactions, provide a crucial supportive role. The polarity of the ethanol molecule, stemming from its hydroxyl group, dictates the strength of these interactions, shaping its macroscopic properties.
The Macroscopic Manifestations: How Intermolecular Forces Shape Ethanol’s Properties
Having explored the individual intermolecular forces at play within ethanol, it’s time to connect these microscopic interactions to the macroscopic properties we observe in the laboratory and in everyday life. The strength and type of these forces directly influence characteristics such as boiling point, solubility, and viscosity, shaping ethanol’s behavior in predictable and often crucial ways.
Boiling Point: A Measure of Intermolecular Strength
Boiling point serves as a readily observable indicator of the strength of intermolecular forces within a liquid. It represents the temperature at which a liquid transitions to a gaseous state, requiring sufficient energy to overcome the attractive forces holding the molecules together. Ethanol’s boiling point, at 78.37 °C (173.07 °F; 351.52 K), is significantly higher than that of other organic molecules with similar molecular weights, such as diethyl ether (34.6 °C) or butane (-0.5 °C).
This elevated boiling point is primarily attributed to the presence of strong hydrogen bonding between ethanol molecules.
The Role of Hydrogen Bonding
The hydroxyl group (-OH) in ethanol allows each molecule to form hydrogen bonds with several neighboring molecules. These bonds, substantially stronger than typical Van der Waals forces, create a network of intermolecular attractions that require a considerable amount of energy to disrupt.
In contrast, molecules like diethyl ether, which lack the capacity for significant hydrogen bonding, exhibit much lower boiling points. While diethyl ether possesses dipole-dipole interactions, they are not as strong or as extensive as the hydrogen bonding network in ethanol. Similarly, the nonpolar butane, relying only on weaker London dispersion forces, boils at a temperature well below zero degrees Celsius.
Implications of a Higher Boiling Point
Ethanol’s relatively high boiling point has important practical implications. It makes ethanol a useful solvent in applications where volatility needs to be controlled.
It also influences its behavior in mixtures, such as alcoholic beverages, where the rate of evaporation affects aroma and flavor profiles.
Solubility: A Tale of Two Affinities
Solubility, the ability of a substance to dissolve in a solvent, is another property deeply influenced by intermolecular forces. Ethanol exhibits a remarkable ability to dissolve in water in all proportions, meaning it is completely miscible. This miscibility stems from the ability of ethanol to form hydrogen bonds with water molecules.
Hydrogen Bonds Between Ethanol and Water
Water, like ethanol, is a polar molecule capable of forming extensive hydrogen bonds. When ethanol is introduced into water, the hydroxyl group on the ethanol molecule readily forms hydrogen bonds with the surrounding water molecules, effectively integrating itself into the water’s hydrogen bonding network.
This mutual affinity, driven by the formation of hydrogen bonds, allows ethanol to disperse evenly throughout the water, resulting in a homogeneous solution.
Polarity and Solubility
The polarity of ethanol also plays a role in its solubility. While the ethyl group (C2H5) is nonpolar, the presence of the hydroxyl group imparts a degree of polarity to the overall molecule. This amphiphilic character – possessing both polar and nonpolar regions – allows ethanol to interact with both polar (water) and nonpolar substances to some extent, enhancing its versatility as a solvent.
Comparing to other substances
In contrast, nonpolar substances like oil are immiscible with water because they cannot form favorable interactions with water molecules. Their interactions are limited to weak Van der Waals forces, which are insufficient to overcome the strong hydrogen bonding between water molecules.
Connecting Intermolecular Forces to Macroscopic Behavior
The examples of boiling point and solubility illustrate a fundamental principle: the macroscopic properties of a substance are a direct consequence of the intermolecular forces acting at the molecular level. Understanding these forces allows us to predict and explain the behavior of ethanol in various contexts, from its use as a fuel and solvent to its role in biological systems.
The interplay between hydrogen bonding, Van der Waals forces, and molecular polarity dictates not only how ethanol molecules interact with each other but also how they interact with other substances, shaping its physical and chemical properties in profound ways.
Ethanol’s Secret Forces: Frequently Asked Questions
Here are some common questions about the intermolecular forces at play within ethanol and how they impact its properties.
What makes ethanol a liquid at room temperature?
Ethanol is a liquid because of the strength of its intermolecular forces. These forces, including hydrogen bonding (the strongest type present in ethanol), dipole-dipole interactions, and London dispersion forces, hold the ethanol molecules together tightly enough to prevent them from becoming a gas at typical room temperatures.
How does hydrogen bonding affect ethanol’s properties?
Hydrogen bonding, a specific type of dipole-dipole interaction, is a major contributor to ethanol’s higher boiling point compared to similar-sized molecules. The strong attraction between the hydrogen atom in the -OH group of one ethanol molecule and the oxygen atom of another necessitates more energy to overcome the intermolecular forces in ethanol and transition it into a gaseous state.
Does ethanol only have hydrogen bonds between its molecules?
No, hydrogen bonding is the dominant force, but it’s not the only one. Ethanol also exhibits dipole-dipole interactions because of the polar O-H bond. Additionally, all molecules, including ethanol, experience London dispersion forces, which are temporary, fluctuating attractions caused by instantaneous dipoles due to electron movement. All of these intermolecular forces in ethanol contribute to its overall properties.
How do intermolecular forces influence ethanol’s ability to dissolve other substances?
The polarity of ethanol, largely due to the -OH group and the resulting hydrogen bonding, makes it a good solvent for both polar and nonpolar substances. Polar substances dissolve well in ethanol because of similar intermolecular attractions. Nonpolar substances can also dissolve to some extent due to weaker London dispersion forces and some dipole-induced dipole interactions, giving ethanol its versatility as a solvent.
So, that’s the lowdown on ethanol’s hidden attractions! Hopefully, you’ve got a better grasp on the intermolecular forces in ethanol now. Play around with these ideas, and let me know if anything cool comes to mind!



