Ionic compounds, such as sodium chloride (NaCl), possess crystalline structures essential to understanding their behavior in aqueous solutions. Water’s polarity, a fundamental property, plays a crucial role in facilitating the separation of ions. The dissolution process, influenced by factors like temperature, illustrates the importance of dissolution of ionic compounds in water for various biological and industrial applications. Electrolyte solutions, formed through this dissolution, conduct electricity, highlighting their significance in biological systems and electrochemical reactions.
Imagine a world without the spark of a thought, the twitch of a muscle, or the simple act of maintaining hydration. These seemingly disparate processes are intimately linked by a fundamental interaction: the dissolution of ionic compounds in water. At the heart of this phenomenon lies a partnership between charged particles, ions, and the ubiquitous solvent, water, a collaboration that underpins countless biological and chemical processes.
The Spark of Life: Ions in Action
Consider the crucial role of sodium chloride (NaCl), common table salt, in nerve function. Nerve impulses, the electrical signals that allow us to think, feel, and move, are generated by the precisely controlled flow of sodium (Na+) and potassium (K+) ions across nerve cell membranes. This intricate dance of ions, orchestrated in a watery environment, is essential for communication within the nervous system. Without the ability of water to dissolve NaCl and other ionic compounds, this critical process would grind to a halt, and life as we know it would cease to exist.
Ionic Compounds: Building Blocks of Charge
Ionic compounds are formed through the transfer of electrons between atoms, creating positively charged ions (cations) and negatively charged ions (anions). These ions are then held together by strong electrostatic forces, forming a crystal lattice structure. Common examples include NaCl, potassium chloride (KCl), and calcium chloride (CaCl2), each playing a vital role in various biological and industrial applications.
Water: The Universal Solvent
Water’s remarkable ability to dissolve ionic compounds stems from its unique molecular structure. The bent shape of the water molecule and the unequal sharing of electrons between oxygen and hydrogen atoms create a polar molecule, with a slightly negative charge on the oxygen atom and slightly positive charges on the hydrogen atoms. This polarity allows water molecules to interact strongly with ions, effectively pulling them apart from the crystal lattice and dispersing them throughout the solution.
A Critical Interaction: Dissolution and Its Significance
The dissolution of ionic compounds in water is not merely a chemical curiosity; it is a fundamental process that sustains life and powers a wide range of applications. From maintaining the delicate balance of electrolytes in our bodies to enabling essential chemical reactions in industry, the interaction between ions and water is a cornerstone of our world. This article will delve into the intricacies of this partnership, exploring the mechanisms, factors, and applications that make it so vital.
Imagine a world without the spark of a thought, the twitch of a muscle, or the simple act of maintaining hydration. These seemingly disparate processes are intimately linked by a fundamental interaction: the dissolution of ionic compounds in water. At the heart of this phenomenon lies a partnership between charged particles, ions, and the ubiquitous solvent, water, a collaboration that underpins countless biological and chemical processes.
As we delve deeper into this collaboration, it’s crucial to first understand the individual players involved. On one side, we have water, the universal solvent, and on the other, ionic compounds, the silent architects of countless reactions. Let’s begin by shining a spotlight on these essential building blocks of chemistry.
Understanding the Key Players: Ionic Compounds
Ionic compounds represent a fundamental class of chemical substances, distinguished by their unique mode of formation and characteristic properties. These compounds, ubiquitous in both natural and synthetic systems, arise from the intricate dance of electron transfer and electrostatic attraction between atoms.
The Formation of Ionic Bonds
At the heart of ionic compound formation lies the transfer of electrons between atoms with significantly different electronegativities. Typically, this involves a metal atom, which readily loses one or more electrons to achieve a stable electron configuration, and a nonmetal atom, which readily gains electrons to complete its valence shell.
This transfer results in the creation of charged particles known as ions. Atoms that lose electrons become positively charged ions, or cations, while those that gain electrons become negatively charged ions, or anions.
The driving force behind ionic bond formation is the quest for stability. Atoms "want" to achieve a noble gas electron configuration, which is particularly stable.
Once formed, these oppositely charged ions are drawn together by the irresistible force of electrostatic attraction. This attraction, governed by Coulomb’s law, is proportional to the magnitude of the charges and inversely proportional to the square of the distance between them. The resulting electrostatic force is what holds the ions together in a crystal lattice.
Characteristic Properties
The strong electrostatic forces between ions in the crystal lattice bestow ionic compounds with several distinguishing characteristics.
One notable property is their high melting point. Substantial energy is required to overcome the strong electrostatic attractions and disrupt the orderly arrangement of ions in the lattice. As a result, ionic compounds typically exist as solids at room temperature and require high temperatures to melt.
Another hallmark of ionic compounds is their brittleness. When subjected to mechanical stress, the regular arrangement of ions can be disrupted, causing ions of like charge to come into close proximity. The resulting electrostatic repulsion leads to cleavage along crystal planes, resulting in brittle fracture.
Common Examples
Ionic compounds are abundant in our daily lives and play critical roles in various applications.
Sodium chloride (NaCl), or common table salt, is perhaps the most familiar example. It is essential for human health and is widely used in food preservation and flavoring.
Potassium chloride (KCl) is another vital ionic compound, playing a crucial role in maintaining electrolyte balance in the body and is often used as a salt substitute.
Calcium chloride (CaCl2) finds applications in various industries, including road de-icing, food processing, and medicine. Its ability to absorb moisture from the air also makes it useful as a drying agent. These are just a few examples of the vast array of ionic compounds that exist, each with its unique properties and applications.
Understanding the Key Players: Water
Just as an architect relies on specific materials with unique properties, the dissolution of ionic compounds hinges on the remarkable characteristics of water. Water’s seemingly simple molecular structure belies a complexity that makes it uniquely suited to interact with and dissolve ionic compounds. To understand why water is such an effective solvent, we need to delve into its polarity and hydrogen bonding capabilities.
The Polar Nature of Water
The water molecule (H₂O) is not linear but rather bent, with an angle of approximately 104.5° between the two hydrogen atoms. This bent geometry is crucial to understanding its polarity.
Oxygen is significantly more electronegative than hydrogen. This means it attracts electrons more strongly, leading to an unequal sharing of electrons in the O-H bonds.
The oxygen atom carries a partial negative charge (δ-), while each hydrogen atom carries a partial positive charge (δ+). This separation of charge creates a dipole moment, making the water molecule polar.
This polarity is not just a molecular curiosity; it’s the key to water’s solvent prowess.
The Significance of Hydrogen Bonding
Water’s polarity gives rise to another critical property: hydrogen bonding. A hydrogen bond is an attractive force between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another.
These bonds are relatively weak compared to covalent bonds, but their sheer number makes them incredibly significant.
Each water molecule can form hydrogen bonds with up to four other water molecules, creating a dynamic network.
This network is responsible for many of water’s unique properties, including its relatively high boiling point and surface tension.
Water as the "Universal Solvent"
Water’s ability to dissolve a wide range of substances has earned it the moniker "universal solvent," although, more accurately, it is an excellent solvent for polar and ionic compounds.
Its polarity allows it to interact favorably with other polar molecules.
Critically, the polarity of water facilitates its interaction with ions, the charged components of ionic compounds. The partially negative oxygen atoms in water are attracted to cations (positively charged ions), while the partially positive hydrogen atoms are attracted to anions (negatively charged ions).
This interaction, known as ion-dipole interaction, is the driving force behind the dissolution of ionic compounds, a process we will explore in greater detail later. Water’s ability to form hydration shells around ions effectively isolates them and allows them to disperse throughout the solution, disrupting the strong ionic bonds that hold the crystalline lattice together.
Therefore, water’s role is more than just a medium; it is an active participant in the dissolution process.
The Dissolution Process: A Step-by-Step Guide
Having explored the individual characteristics of ionic compounds and water, we now turn our attention to the central event: the dissolution process. Understanding this process requires a detailed examination of how water molecules interact with ions at the surface of the crystal lattice and how these interactions ultimately lead to the separation and dispersal of ions within the aqueous medium.
Ion-Dipole Interactions: The Key to Dissolution
At the heart of the dissolution process lies the ion-dipole interaction. This interaction is the attractive force that arises between an ion and a polar molecule.
In the case of ionic compounds dissolving in water, the partially positive hydrogen atoms (δ+) of water molecules are attracted to negatively charged anions. Conversely, the partially negative oxygen atoms (δ-) are attracted to positively charged cations.
This electrostatic attraction is crucial for initiating the breakdown of the ionic lattice. The orientation of water molecules around the ions is highly specific, maximizing the attractive forces and minimizing repulsive ones.
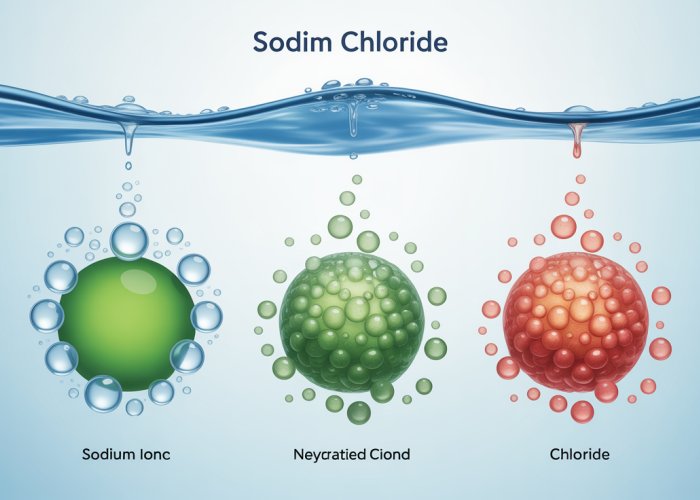
Hydration Shells: Stabilizing the Ions
As water molecules cluster around an ion, they form what is known as a hydration shell. This shell is a sphere of water molecules surrounding the ion, effectively isolating it from other ions in the solution.
The number of water molecules in a hydration shell depends on the size and charge density of the ion. Smaller ions with higher charges tend to have larger hydration shells.
The formation of hydration shells is an exothermic process, releasing energy that contributes to the overall energy balance of dissolution. These shells not only stabilize the ions in solution but also prevent them from recombining to reform the original ionic compound.
Breaking the Ionic Lattice: Overcoming the Odds
For dissolution to occur, the attractive forces between water molecules and ions must be strong enough to overcome the lattice energy of the ionic compound.
Lattice energy is the energy required to completely separate one mole of a solid ionic compound into its gaseous ions. Ionic compounds with high lattice energies are generally less soluble because more energy is required to break apart the crystal structure.
The ion-dipole interactions provided by water molecules effectively "pry" the ions away from the lattice, weakening the ionic bonds.
The Importance of Solvent Properties
Water’s high dielectric constant is another crucial property that aids in dissolution. The dielectric constant measures a solvent’s ability to reduce the force of attraction between oppositely charged ions. Water’s high dielectric constant weakens the electrostatic attraction between ions, making it easier for them to separate and disperse.
The Role of Entropy: Disorder Reigns
While the energetic considerations are important, the entropy also plays a vital role in driving the dissolution process. Entropy is a measure of disorder or randomness in a system.
Dissolution leads to an increase in entropy as the highly ordered crystal lattice is broken down, and the ions become dispersed throughout the solution. This increase in entropy favors the dissolution process, as systems tend to move towards states of higher disorder.
Even if the energy required to break the ionic lattice is significant, the increase in entropy can sometimes be enough to make the dissolution process spontaneous. This is because systems tend to move towards a state of lower energy and higher entropy, balancing these two factors.
The hydration shells formed during dissolution not only stabilize individual ions but also enable their crucial function as electrolytes. Understanding the nature and biological importance of electrolytes is essential for appreciating the full significance of ionic compound dissolution.
Electrolytes and Their Importance in Biological Systems
Electrolytes are substances that, when dissolved in a solvent like water, dissociate into ions and are able to conduct electrical current. These ions, liberated from ionic compounds during dissolution, are the fundamental building blocks of electrolytes. In essence, the process of dissolving ionic compounds creates the very foundation for electrolyte solutions.
Defining Electrolytes: The Charge Carriers of Life
The term "electrolyte" specifically refers to the solution formed after an ionic compound dissolves and dissociates into its constituent ions. These ions, being charged particles, are capable of carrying an electrical current. This ability to conduct electricity is what distinguishes an electrolyte solution from a non-electrolyte solution (e.g., sugar dissolved in water).
A critical distinction to remember is that it is the free ions resulting from dissolution that give electrolytes their characteristic properties. The concentration of these ions directly influences the electrolyte’s conductivity: the higher the ion concentration, the greater the conductivity. This conductivity is the foundation for various biological processes.
The Key Players: Sodium, Potassium, and Calcium
While various ions can contribute to electrolyte balance, a few stand out due to their crucial roles in biological systems: sodium (Na+), potassium (K+), and calcium (Ca2+). These ions are actively involved in a myriad of physiological processes, ranging from nerve impulse transmission to muscle contraction and maintaining fluid balance.
Nerve Impulses: The Sodium-Potassium Symphony
Nerve impulse transmission, the rapid communication system of the nervous system, relies heavily on the controlled movement of Na+ and K+ ions across nerve cell membranes.
The process involves the creation of an electrochemical gradient, where a difference in ion concentration and electrical potential exists between the inside and outside of the nerve cell.
This gradient is maintained by the sodium-potassium pump, which actively transports Na+ ions out of the cell and K+ ions into the cell, requiring energy in the form of ATP. When a nerve cell is stimulated, these ion channels open, allowing a rapid influx of Na+ ions into the cell.
This influx depolarizes the cell membrane, generating an electrical signal that propagates along the nerve fiber. Subsequently, K+ channels open, allowing K+ ions to flow out of the cell, repolarizing the membrane and restoring the resting potential. The orchestrated movement of these ions creates the nerve impulse, a signal that allows us to perceive, think, and react.
Muscle Contraction: Calcium’s Orchestration
Muscle contraction, the mechanism enabling movement, is critically dependent on calcium ions (Ca2+). When a muscle fiber receives a signal from a motor neuron, it triggers the release of Ca2+ ions from intracellular storage sites. These calcium ions then bind to specific proteins within the muscle fiber, initiating a cascade of events that ultimately leads to muscle contraction.
Ca2+ ions bind to troponin, a protein associated with actin filaments. This binding causes a conformational change in troponin, which in turn moves tropomyosin, another protein that blocks the binding sites on actin for myosin. With tropomyosin moved aside, myosin heads can now bind to actin, forming cross-bridges.
The myosin heads then pull on the actin filaments, causing them to slide past the myosin filaments, shortening the muscle fiber and generating force. This process requires ATP, which provides the energy for the myosin heads to detach from actin and re-cock for another cycle.
The removal of Ca2+ ions from the cytoplasm, by pumping them back into intracellular storage, causes the muscle to relax. Without calcium present to bind to troponin, tropomyosin returns to its blocking position, preventing myosin from binding to actin.
General Biological Importance: Maintaining Homeostasis
Beyond nerve impulse transmission and muscle contraction, electrolytes play a crucial role in maintaining overall homeostasis, the body’s ability to maintain a stable internal environment. They contribute to regulating fluid balance, blood pH, and osmotic pressure. Disruptions in electrolyte balance can have severe consequences, leading to a range of health problems.
Proper hydration, achieved through the consumption of fluids containing electrolytes, is essential for maintaining these vital functions. The dissolution of ionic compounds in these fluids provides the necessary ions to replenish those lost through sweat, urine, and other bodily processes. The interconnectedness of ionic compound dissolution and electrolyte balance is paramount for maintaining health and sustaining life.
The remarkable ability of ionic compounds to dissolve, thereby creating electrolytes that power essential biological and industrial processes, makes the question of what influences this solubility particularly pertinent. While the nature of the solvent and solute plays a fundamental role, external factors can also significantly impact the extent to which an ionic compound will dissolve in water.
Factors Influencing Solubility
The solubility of ionic compounds, or the extent to which they dissolve in a solvent like water, isn’t a fixed property. Instead, it’s a dynamic characteristic influenced by several factors. While the chemical properties of both the solute (the ionic compound) and the solvent (typically water) set the stage, external conditions like temperature and, to a lesser extent, pressure, can shift the equilibrium of dissolution. Understanding these influences is crucial for optimizing various chemical and biological processes.
The Predominant Role of Temperature
Temperature exerts a significant influence on the solubility of most ionic compounds in water. Generally, increasing the temperature of the solvent increases the solubility of solids. This is because higher temperatures provide more kinetic energy to both the solvent and solute molecules.
Endothermic vs. Exothermic Dissolution
The effect of temperature on solubility is directly related to the enthalpy change (ΔH) of the dissolution process.
For endothermic dissolution (ΔH > 0), where energy is absorbed from the surroundings, increasing the temperature favors the forward reaction (dissolution). This leads to a higher solubility.
Conversely, for exothermic dissolution (ΔH < 0), where energy is released, increasing the temperature favors the reverse reaction (precipitation). This decreases the solubility.
Put simply, if dissolving the ionic compound requires energy, adding heat will help it dissolve more. If dissolving the ionic compound releases energy, adding heat will make it dissolve less.
Practical Examples
Consider potassium nitrate (KNO3), an ionic compound commonly used in fertilizers. Its dissolution in water is endothermic. As a result, its solubility increases dramatically with increasing temperature. This means you can dissolve far more KNO3 in hot water than in cold water.
However, some ionic compounds, like cerium(III) sulfate (Ce2(SO4)3), exhibit a decrease in solubility with increasing temperature. This indicates an exothermic dissolution process.
The Underlying Mechanism
The increase in solubility with temperature observed for most ionic compounds can be attributed to the increased kinetic energy of the water molecules. This increased energy allows water molecules to more effectively overcome the lattice energy holding the ions together in the solid state. The more vigorous movement of water molecules also facilitates the formation and stabilization of hydration shells around the separated ions, further promoting dissolution.
The Limited Impact of Pressure
While pressure significantly affects the solubility of gases in liquids, its impact on the solubility of solid ionic compounds in water is generally considered negligible. This is because liquids and solids are virtually incompressible.
Changes in pressure, even substantial ones, don’t significantly alter the volume or structure of the solid or the liquid water. Therefore, the interactions between ions and water molecules remain largely unaffected.
Exceptions and Considerations
While pressure’s influence is usually minimal, there might be slight effects under extremely high pressures. These effects, however, are rarely encountered in typical laboratory or biological settings.
Moreover, if the dissolution process involves the formation of gaseous species as a byproduct, then pressure can indirectly influence the overall process. However, this is not a direct effect on the solubility of the ionic compound itself.
In conclusion, while other factors might play a minor role, temperature remains the most significant and readily manipulated factor influencing the solubility of ionic compounds in water. The relationship between temperature and solubility is dictated by whether the dissolution process is endothermic or exothermic, providing a key handle for controlling the extent of dissolution in various applications.
Real-World Applications of Ionic Compound Dissolution
Having explored the factors governing ionic compound solubility, it becomes clear that this process isn’t just a textbook phenomenon. Its influence resonates deeply throughout our lives, underpinning crucial aspects of health, agriculture, and numerous industrial processes. Let’s delve into some specific examples that highlight the tangible importance of ionic compound dissolution.
Health and Medicine: The Electrolyte Imperative
The significance of ionic compound dissolution is perhaps most evident in the realm of health and medicine, particularly concerning hydration and electrolyte balance. Our bodies rely on a precise concentration of ions like sodium (Na+), potassium (K+), chloride (Cl-), calcium (Ca2+), and magnesium (Mg2+) for a multitude of physiological functions.
Electrolytes for Hydration: Replenishing What’s Lost
During physical exertion, illness (vomiting/diarrhea), or even just through daily activities, we lose fluids and, consequently, electrolytes. Drinks designed for hydration, such as sports drinks and oral rehydration solutions (ORS), capitalize on the dissolution of ionic compounds to replenish these lost electrolytes.
These beverages contain carefully formulated amounts of salts like sodium chloride (NaCl) and potassium chloride (KCl), which readily dissolve in water. This dissolution process releases the essential ions, enabling the body to restore proper fluid balance and maintain critical functions such as nerve impulse transmission and muscle contraction. The addition of glucose (sugar) to ORS further aids in electrolyte absorption within the small intestine.
Agriculture: Nourishing Crops with Dissolved Ions
Ionic compounds are also indispensable in agriculture, serving as the foundation for many fertilizers. Plants require a range of nutrients, including nitrogen, phosphorus, and potassium, to thrive.
These elements are often supplied in the form of ionic compounds that dissolve in water, allowing plants to absorb them through their roots.
Essential Plant Nutrients
For instance, ammonium nitrate (NH4NO3), potassium nitrate (KNO3), and various phosphate salts are commonly used fertilizers. When these ionic compounds dissolve in the soil’s moisture, they release ammonium (NH4+), nitrate (NO3-), potassium (K+), and phosphate (PO43-) ions, respectively.
These ions are then readily available for uptake by plants, providing them with the essential building blocks for growth, development, and overall health.
Industry: The Dissolution Backbone of Chemical Processes
Beyond health and agriculture, the dissolution of ionic compounds is critical in a wide array of industrial chemical processes. Many chemical reactions require reactants to be in solution, allowing for efficient mixing and interaction at the molecular level.
Examples in Industrial Chemistry
Consider, for example, the production of chlorine gas (Cl2) and sodium hydroxide (NaOH) through the electrolysis of brine (concentrated sodium chloride solution). The dissolution of NaCl in water is a prerequisite for this process, as it creates the necessary mobile ions to conduct electricity and facilitate the electrochemical reactions.
Similarly, many metal extraction and refining processes rely on dissolving metal ores in aqueous solutions containing ionic compounds.
The dissolved ions allow for selective separation and purification of the desired metals, highlighting the indispensable role of ionic compound dissolution in numerous industrial applications.
FAQs: Ionic Compounds & Water’s Secret
Hopefully, this clarifies some common questions about ionic compounds and water!
Why is water so good at dissolving ionic compounds?
Water is a polar molecule. Its slight positive and negative charges are attracted to the positive and negative ions in ionic compounds. This attraction overcomes the forces holding the ions together, pulling them apart and dispersing them throughout the water.
What happens to ionic compounds when they dissolve in water?
When an ionic compound dissolves, it dissociates into individual ions. For example, NaCl (table salt) breaks down into Na+ and Cl- ions. These ions are then surrounded by water molecules, stabilizing them in solution.
Why is the dissolution of ionic compounds in water important?
The importance of dissolution of ionic compounds in water cannot be overstated. It allows ions to be transported and utilized in biological systems. Many essential processes like nerve impulses and muscle contractions rely on ionic solutions within our bodies.
Are all ionic compounds equally soluble in water?
No. Solubility depends on the strength of the attraction between the ions in the compound versus the attraction between the ions and water molecules. Some ionic compounds are highly soluble, while others are practically insoluble.
So, now you know a little more about why the importance of dissolution of ionic compounds in water really matters! Hope you found it helpful. Keep exploring, and thanks for stopping by!