The Bohr Model, developed by Niels Bohr, provides a foundational framework for understanding atomic structure. Specifically, the model’s application to the nitrogen atom bohr elucidates electron energy levels and orbital configurations. Understanding this requires grasping principles of quantum mechanics. Analysis of spectral lines, often performed using spectroscopy tools, provides empirical validation of the Bohr model’s predictions about the nitrogen atom bohr and similar atomic systems. The model itself offers a visualizable yet simplified representation of atomic behavior, which is crucial for students learning introductory chemistry concepts. Furthermore, many educational resources, including those provided by institutions and groups such as the Royal Society of Chemistry, assist in decoding the complexities associated with the nitrogen atom bohr within the Bohr model.
Understanding atomic structure is fundamental to grasping the behavior of matter and the universe itself.
From the interactions of molecules in biological systems to the creation of new materials in industrial processes, the arrangement and behavior of atoms dictate the properties we observe.
The Ubiquitous Nitrogen Atom
Among the elements, nitrogen holds a particularly vital role. It is a cornerstone of life, forming the backbone of amino acids, the building blocks of proteins, and nucleic acids, the carriers of genetic information.
In the realm of chemistry, nitrogen is a crucial component of numerous compounds, including fertilizers, polymers, and pharmaceuticals.
Industrially, nitrogen is employed in the production of ammonia, a key ingredient in fertilizers, and as a cooling agent in various processes.
Its inert nature also makes it valuable as a protective atmosphere in electronics manufacturing and food packaging.
The Bohr Model: A Stepping Stone to Atomic Understanding
To unravel the complexities of atomic structure, scientists have developed various models, each offering a unique perspective.
Among these, the Bohr model stands out as a simplified, yet foundational, representation of the atom.
Proposed by Niels Bohr in 1913, this model postulates that electrons orbit the nucleus in specific, quantized energy levels, much like planets orbiting the sun.
While superseded by more sophisticated quantum mechanical models, the Bohr model remains a valuable tool for introducing the basic concepts of atomic structure and electron behavior.
Article Objective
This article aims to explain the structure of the nitrogen atom using the Bohr model.
By examining the arrangement of electrons in nitrogen according to the Bohr model’s principles, we can gain a clearer understanding of its chemical properties and its role in various scientific fields.
This simplified approach will provide a solid foundation for further exploration into the more complex world of quantum mechanics and advanced atomic theory.
Understanding atomic structure is fundamental to grasping the behavior of matter and the universe itself.
From the interactions of molecules in biological systems to the creation of new materials in industrial processes, the arrangement and behavior of atoms dictate the properties we observe.
The Ubiquitous Nitrogen Atom
Among the elements, nitrogen holds a particularly vital role. It is a cornerstone of life, forming the backbone of amino acids, the building blocks of proteins, and nucleic acids, the carriers of genetic information.
In the realm of chemistry, nitrogen is a crucial component of numerous compounds, including fertilizers, polymers, and pharmaceuticals.
Industrially, nitrogen is employed in the production of ammonia, a key ingredient in fertilizers, and as a cooling agent in various processes.
Its inert nature also makes it valuable as a protective atmosphere in electronics manufacturing and food packaging.
The Bohr Model: A Stepping Stone to Atomic Understanding
To unravel the complexities of atomic structure, scientists have developed various models, each offering a unique perspective.
Among these, the Bohr model stands out as a simplified, yet foundational, representation of the atom.
Proposed by Niels Bohr in 1913, this model postulates that electrons orbit the nucleus in specific, quantized energy levels, much like planets orbiting the sun.
While superseded by more sophisticated quantum mechanical models, the Bohr model remains a valuable tool for introducing the basic concepts of atomic structure and electron behavior.
Article Objective
This article aims to explain the structure of the nitrogen atom using the Bohr model.
By examining the arrangement of its constituent particles and the behavior of its electrons, we can gain a deeper appreciation for the properties and reactivity of this essential element.
However, before we delve into the specifics of nitrogen, it’s important to lay a foundation by understanding the fundamental principles of atomic structure and the key ideas behind the Bohr model itself.
The Fundamentals: Atoms and the Bohr Model
To truly appreciate the application of the Bohr model to the nitrogen atom, we must first revisit the core concepts of atomic structure and the model’s underlying principles. This section serves as a crucial refresher, establishing the necessary groundwork for a comprehensive understanding.
Atomic Structure: The Basic Building Blocks
At the heart of all matter lies the atom, the smallest unit of an element that retains its chemical properties.
Atoms are not indivisible; rather, they are composed of even smaller subatomic particles: protons, neutrons, and electrons.
-
Protons, located in the nucleus, carry a positive electrical charge. The number of protons defines the element; for instance, all atoms with seven protons are nitrogen atoms.
-
Neutrons, also residing in the nucleus, have no electrical charge (they are neutral). Neutrons contribute to the atom’s mass and influence its stability.
-
Electrons, much smaller than protons and neutrons, carry a negative electrical charge and orbit the nucleus in specific energy levels or shells.
The arrangement and behavior of these subatomic particles dictate the atom’s chemical properties and how it interacts with other atoms.
The Bohr Model: A Simplified Representation
The Bohr model, introduced by Niels Bohr in 1913, offered a revolutionary, albeit simplified, picture of atomic structure.
It proposed that electrons orbit the nucleus in specific, quantized energy levels, much like planets orbiting the sun.
These energy levels are often referred to as electron shells or orbitals.
Key postulates of the Bohr model include:
-
Electrons can only occupy specific energy levels, corresponding to discrete distances from the nucleus.
-
Electrons can jump from one energy level to another by absorbing or emitting energy in the form of photons.
-
The energy of the emitted or absorbed photon is equal to the difference in energy between the two energy levels.
While not entirely accurate, the Bohr model provided a valuable framework for understanding the quantized nature of electron energy and the relationship between electron transitions and light emission.
Niels Bohr: The Architect of the Model
Niels Bohr (1885-1962), a Danish physicist, played a pivotal role in the development of atomic theory.
His model, based on Rutherford’s nuclear model and Planck’s quantum theory, marked a significant departure from classical physics.
Bohr’s work earned him the Nobel Prize in Physics in 1922 and laid the foundation for future advancements in quantum mechanics.
His insights into atomic structure revolutionized our understanding of matter and paved the way for numerous technological innovations.
Limitations of the Bohr Model
Despite its historical significance, the Bohr model has limitations. It accurately predicts the behavior of hydrogen, an atom with only one electron.
However, it fails to adequately explain the spectra of more complex atoms with multiple electrons.
The Bohr model also violates the Heisenberg Uncertainty Principle, which states that it is impossible to know both the position and momentum of an electron with perfect accuracy.
Furthermore, it does not account for the wave-particle duality of electrons or the shapes of atomic orbitals.
Acknowledging these limitations is crucial, as it sets the stage for understanding the need for more sophisticated quantum mechanical models that provide a more complete and accurate description of atomic structure.
The Bohr Model: A Stepping Stone to Atomic Understanding
To unravel the complexities of atomic structure, scientists have developed various models, each offering a unique perspective.
Among these, the Bohr model stands out as a simplified, yet foundational, representation of the atom.
With the fundamentals of atoms and the Bohr model now established, it’s time to turn our attention to the star of our show: nitrogen. This element, so vital yet so often overlooked, plays a critical role in countless processes, both natural and industrial. To truly understand its behavior, we must first examine its intrinsic properties.
Nitrogen: An Elemental Overview
Defining the Nitrogen Atom
At the heart of understanding any element lies its atomic number and atomic mass. These two numbers serve as the element’s unique identifiers, dictating its place in the periodic table and its fundamental properties.
For nitrogen, the atomic number is 7. This seemingly simple number holds profound implications for the element’s identity and behavior.
But what exactly does the atomic number tell us?
The Significance of the Atomic Number
The atomic number represents the number of protons found in the nucleus of an atom.
In the case of nitrogen, the presence of seven protons is the defining characteristic that distinguishes it from all other elements.
An atom with six protons is carbon; one with eight is oxygen. Change the number of protons, and you change the element itself.
Furthermore, in a neutral atom, the number of protons is equal to the number of electrons orbiting the nucleus.
This equality is crucial, as it dictates the atom’s electrical neutrality and influences how it interacts with other atoms to form molecules.
The atomic number, therefore, is not merely a label but a fundamental descriptor that governs the very essence of an element.
Atomic Mass: A Glimpse into the Nucleus
While the atomic number defines an element, the atomic mass provides insight into the composition of its nucleus.
The atomic mass is essentially the total mass of protons and neutrons found within the nucleus.
Nitrogen’s most common isotope, Nitrogen-14, has an approximate atomic mass of 14 atomic mass units (amu).
This indicates that, in addition to its seven protons, it also contains seven neutrons.
It’s worth noting that elements can exist in multiple isotopic forms, meaning they have the same number of protons but different numbers of neutrons.
While these isotopes may exhibit slightly different physical properties, they remain fundamentally the same element, defined by their atomic number.
Electron Configuration of Nitrogen: Filling the Energy Levels
Having established nitrogen’s identity through its atomic number, we now shift our focus to how its electrons are arranged.
This arrangement, known as the electron configuration, dictates how nitrogen interacts with other elements and forms chemical bonds. Understanding this configuration is key to unlocking nitrogen’s reactivity and role in chemical processes.
Deciphering Electron Configuration
The electron configuration describes the distribution of electrons among the various energy levels and sublevels within an atom. For nitrogen, with its seven electrons, understanding this arrangement reveals how these electrons occupy specific orbitals around the nucleus.
The Bohr model, while simplified, provides a valuable framework for visualizing these energy levels as distinct "shells" surrounding the nucleus.
Energy Levels and Electron Distribution
Electrons do not orbit the nucleus randomly; instead, they reside in specific energy levels, often denoted by the principal quantum number n (n = 1, 2, 3, and so on).
Each energy level can accommodate a limited number of electrons: the first level (n=1) holds a maximum of two electrons, while the second level (n=2) can hold up to eight.
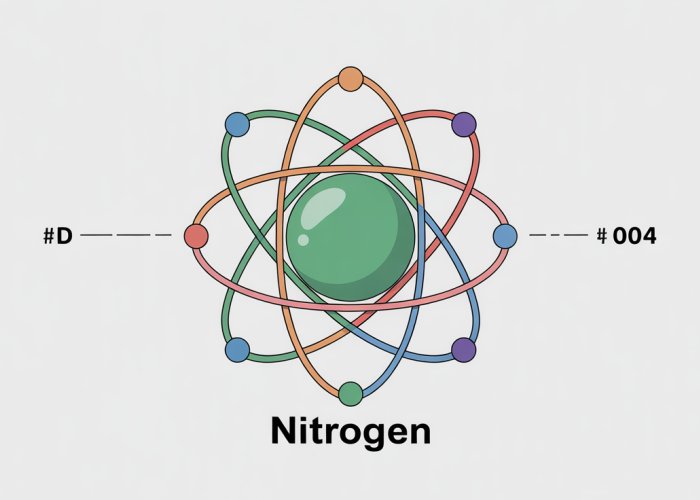
Nitrogen, with its seven electrons, has two electrons in the first energy level and five in the second. This arrangement is commonly written as 1s²2s²2p³, a notation that indicates the number of electrons in each sublevel (s and p) within the respective energy levels.
The Significance of Valence Electrons
Of particular importance are the valence electrons, which are the electrons in the outermost energy level. These electrons are primarily responsible for an atom’s chemical behavior.
Nitrogen has five valence electrons in its second energy level.
This electron count makes nitrogen highly reactive, as it seeks to achieve a stable octet (eight electrons) in its valence shell through chemical bonding with other atoms. This drive to achieve stability explains nitrogen’s propensity to form strong bonds and participate in diverse chemical reactions.
Valence electrons, residing as they do in the outermost shell, exert a profound influence on how nitrogen interacts with its environment. Their arrangement and availability are the primary determinants of nitrogen’s bonding behavior. But to truly grasp these interactions, a visual representation is invaluable.
Visualizing Nitrogen with the Bohr Model: A Simplified Representation
The Bohr Model, despite its limitations when compared to more advanced quantum mechanical models, offers a remarkably accessible way to visualize the structure of the nitrogen atom.
It allows us to picture the atom as a central nucleus orbited by electrons in distinct, quantized energy levels.
This visualization, while a simplification, provides a crucial stepping stone for understanding more complex atomic behaviors.
The Nitrogen Atom in Orbit
Imagine the nitrogen atom, not as a fuzzy cloud of probability as quantum mechanics suggests, but as a miniature solar system.
At the center lies the nucleus, containing protons and neutrons. Surrounding this nucleus, at fixed distances, are the electron orbitals.
According to the Bohr model, these electrons are confined to specific energy levels, each corresponding to a particular orbit.
Electron Distribution: Shells and Orbitals
Nitrogen, with its seven electrons, populates the first two energy levels. The innermost level, closest to the nucleus, can hold a maximum of two electrons.
These two electrons occupy the 1s orbital, represented in our Bohr model visualization as a circular orbit closest to the nucleus.
The remaining five electrons reside in the second energy level, occupying both the 2s and 2p orbitals.
In our simplified representation, we can picture these five electrons orbiting at a greater distance from the nucleus than the two in the first level.
The Bohr Model and Nitrogen’s Electron Configuration
The electron configuration of nitrogen, written as 1s²2s²2p³, directly translates into our Bohr model visualization. The two electrons in the 1s orbital are represented by two dots or symbols on the innermost orbit.
Similarly, the two electrons in the 2s orbital and the three electrons in the 2p orbitals are depicted on the second orbit.
This visual representation allows us to readily see how the seven electrons are distributed among the available energy levels.
It highlights the presence of five valence electrons in the outermost shell, which are critical to understanding nitrogen’s chemical properties.
Limitations and Advantages of the Bohr Model Visualization
It’s crucial to remember that the Bohr model is a simplified representation. It does not accurately depict the true nature of electron behavior.
Electrons do not, in reality, orbit the nucleus in neat, circular paths. Quantum mechanics reveals that they exist in probability clouds, or orbitals, with more complex shapes and behaviors.
However, the Bohr model visualization offers a significant advantage in its simplicity. It provides an easily understandable mental image of electron arrangement.
This makes it an invaluable tool for introducing the concepts of energy levels and electron configuration, especially for those new to the study of atomic structure.
By visualizing nitrogen with the Bohr model, we gain a foundational understanding of its electronic structure, paving the way for exploring more advanced models and concepts in quantum chemistry.
Valence electrons, residing as they do in the outermost shell, exert a profound influence on how nitrogen interacts with its environment. Their arrangement and availability are the primary determinants of nitrogen’s bonding behavior. But to truly grasp these interactions, a visual representation is invaluable.
Beyond Bohr: Quantum Mechanical Refinements
The Bohr model provides an invaluable entry point for understanding atomic structure. However, it’s crucial to acknowledge its limitations. It’s a stepping stone, not the final destination, in our quest to understand the atom. It paints a picture that, while helpful, is ultimately a simplification of a far more complex reality.
The Quantum Leap: Introducing Quantum Mechanics
Quantum mechanics offers a more accurate and nuanced description of atomic behavior. Instead of electrons orbiting the nucleus in fixed paths, quantum mechanics describes electrons existing in regions of probability known as orbitals.
These orbitals are defined by mathematical functions called wave functions. They give the probability of finding an electron in a specific location around the nucleus.
This is a significant departure from the Bohr model’s well-defined orbits. It introduces the concept of uncertainty and the probabilistic nature of electron location.
Electrons as Probability Clouds
In the quantum mechanical view, electrons are not neatly confined to specific orbits. Rather, they exist as diffuse probability clouds surrounding the nucleus.
The shape and size of these clouds are determined by the solutions to the Schrödinger equation, a cornerstone of quantum mechanics. Different solutions correspond to different energy levels and orbital shapes (s, p, d, f, etc.).
This means that while we can’t pinpoint the exact location of an electron at any given moment, we can describe the region where it is most likely to be found.
A More Realistic Picture
The quantum mechanical model provides a more realistic and comprehensive picture of the nitrogen atom. It accounts for phenomena that the Bohr model cannot explain, such as the fine structure of atomic spectra and the details of chemical bonding.
While the Bohr model serves as a valuable teaching tool. Quantum mechanics is essential for understanding the intricacies of atomic and molecular behavior.
It allows scientists to predict and explain the properties of matter with unprecedented accuracy.
The transition from the Bohr model to quantum mechanics represents a significant leap in our understanding of the atomic world. It underscores the importance of continually refining our models to better reflect the complexities of nature.
Decoding Nitrogen Atom: Bohr Model – FAQs
Here are some frequently asked questions to help you better understand the Bohr model of the nitrogen atom.
What are the key components that make up a nitrogen atom according to the Bohr model?
The Bohr model visualizes the nitrogen atom with a central nucleus containing protons and neutrons, orbited by electrons in specific energy levels or shells. These shells are arranged in increasing distance from the nucleus.
How many electrons does a nitrogen atom bohr model depict in each of its electron shells?
A neutral nitrogen atom has 7 electrons. The Bohr model shows two electrons in the first shell (closest to the nucleus) and five electrons in the second shell.
Is the Bohr model a completely accurate representation of a nitrogen atom?
No, the Bohr model is a simplified representation. It doesn’t fully account for electron behavior or the complex shapes of electron orbitals. It’s useful for understanding basic atomic structure.
What’s the significance of understanding the Bohr model for the nitrogen atom bohr model?
Understanding the Bohr model provides a foundational understanding of atomic structure. It allows visualization of how electrons are arranged around the nucleus and forms a base to explore more complex models.
So, there you have it – a simplified look at the nitrogen atom bohr! Hopefully, this helps you grasp the basics. Keep exploring and never stop questioning!



