Understanding atomic structure is fundamental, and the properties of electrons within each energy level significantly impact chemical behavior. Quantum numbers define the characteristics of these electrons, influencing their interactions with the nucleus. The p sublevel, a crucial component of electron configuration, plays a vital role in bonding. Linus Pauling’s work, particularly his studies on chemical bonding, highlighted the importance of electron distribution within sublevels. Determining the maximum number of electrons in the p sublevel, a concept well-explained by the Aufbau principle, is essential for predicting the stability and reactivity of elements.
Unveiling the Secrets of P Sublevel Electrons
Atoms, the fundamental building blocks of matter, are not indivisible spheres as once imagined, but complex systems teeming with activity. At the heart of this activity are electrons, negatively charged particles that orbit the nucleus in specific patterns. These patterns, governed by the principles of quantum mechanics, dictate how atoms interact with each other to form molecules and, ultimately, the world around us.
The Electron’s Crucial Role
Electrons are the key players in determining the chemical properties of any element. Their arrangement and behavior are what govern how atoms bond, react, and behave in various chemical environments. Understanding the architecture of electrons unlocks the potential to understand complex chemical interactions.
Electron Configuration: The Blueprint of Atomic Behavior
The arrangement of electrons within an atom is described by its electron configuration. This configuration dictates an element’s reactivity, its bonding preferences, and many other crucial characteristics. By deciphering an element’s electron configuration, we gain powerful insights into its expected behavior under various conditions. Electron configuration acts as a blueprint, allowing chemists to predict and understand an atom’s interactions with other atoms.
The P Sublevel: A Deep Dive
This exploration will focus specifically on one particularly significant region within the electron configuration: the p sublevel. The p sublevel, present in the second and higher energy levels of atoms, plays a vital role in determining the properties of a vast number of elements.
Many elements essential to life and technology rely on the behavior of their p sublevel electrons. From carbon to oxygen, the characteristics of these elements are primarily driven by how their p electrons interact. This guide will provide a comprehensive understanding of the p sublevel, its structure, and its influence on chemical behavior. By focusing on the p sublevel, we can gain a deeper appreciation for the intricate dance of electrons that governs the world around us.
Atomic Structure and Electron Configuration Fundamentals
Before we can truly grasp the nuances of the p sublevel and its impact on elemental behavior, we must first establish a firm foundation in the fundamentals of atomic structure and electron configuration. This groundwork will provide the necessary context and vocabulary to navigate the intricacies of quantum mechanics and electron behavior within atoms.
Quantum Mechanics Primer
At the heart of understanding electron behavior lies quantum mechanics. This revolutionary theory, developed in the early 20th century, fundamentally altered our understanding of the universe at the atomic and subatomic levels.
Classical physics, which governs the motion of macroscopic objects, breaks down when applied to electrons. Instead, electrons behave according to the principles of quantum mechanics, where energy is quantized, meaning it can only exist in discrete amounts.
Key Quantum Concepts
Several key concepts are particularly relevant to understanding electron behavior:
- Wave-particle duality: Electrons exhibit both wave-like and particle-like properties.
- Heisenberg’s Uncertainty Principle: It is impossible to simultaneously know both the exact position and momentum of an electron.
- Quantization of Energy: Electrons can only occupy specific energy levels within an atom, akin to standing on specific rungs of a ladder.
These principles dictate that we cannot pinpoint an electron’s exact location, but rather describe its probability of being found in a particular region of space.
Energy Levels and Sublevels
Electrons reside in specific energy levels around the nucleus, designated by the principal quantum number n (n = 1, 2, 3…). Higher values of n indicate higher energy levels and greater distances from the nucleus.
Each principal energy level is further divided into sublevels, denoted by the letters s, p, d, and f. These sublevels represent regions of space with slightly different energy characteristics within a given energy level.
The number of sublevels within a principal energy level is equal to the value of n.
- n = 1 has only one sublevel: s
- n = 2 has two sublevels: s, p
- n = 3 has three sublevels: s, p, d
- n = 4 has four sublevels: s, p, d, f
This hierarchical structure of energy levels and sublevels dictates the possible electron configurations within an atom.
Atomic Orbitals Defined
An atomic orbital is a mathematical function that describes the probability of finding an electron in a specific region of space around the nucleus.
It’s important to remember that an orbital is not a physical path the electron follows, but rather a probability distribution.
Each sublevel consists of one or more atomic orbitals:
- The s sublevel has one spherical orbital.
- The p sublevel has three dumbbell-shaped orbitals.
- The d sublevel has five complex-shaped orbitals.
- The f sublevel has seven even more complex-shaped orbitals.
Therefore, the p sublevel, the focus of our attention, comprises three distinct orbitals, each capable of holding a maximum of two electrons.
Electron Configuration Explained
Electron configuration describes the arrangement of electrons within an atom’s energy levels and sublevels. It is a shorthand notation that specifies which orbitals are occupied by electrons.
Following specific rules, we can predict the electron configuration of an element, which in turn allows us to predict its chemical behavior.
Rules for Filling Orbitals
Several rules govern how electrons fill orbitals:
- Aufbau Principle: Electrons first fill the lowest energy orbitals available.
- Hund’s Rule: Within a sublevel, electrons individually occupy each orbital before any orbital is doubly occupied.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers, implying that each orbital can hold a maximum of two electrons with opposite spins.
The electron configuration is written using a notation that indicates the principal energy level, the sublevel, and the number of electrons in that sublevel. For example, the electron configuration of oxygen (O) is 1s²2s²2p⁴, indicating that it has 2 electrons in the 1s sublevel, 2 electrons in the 2s sublevel, and 4 electrons in the 2p sublevel.
Delving into the P Sublevel: Structure and Capacity
With the groundwork of atomic structure and electron configuration laid, we can now turn our attention to the star of our show: the p sublevel. Understanding its unique structure and electron capacity is crucial for predicting how elements interact and form compounds.
Defining the P Sublevel
The p sublevel is a region within an atom’s electron cloud, characterized by a distinct energy level and spatial distribution. Each principal energy level (n) from n=2 onwards contains a p sublevel, in addition to the s sublevel.
Unlike the s sublevel, which has a spherical shape, the p sublevel possesses a more complex geometry. This difference in shape significantly impacts the chemical behavior of elements containing p electrons.
Visualizing P Orbitals
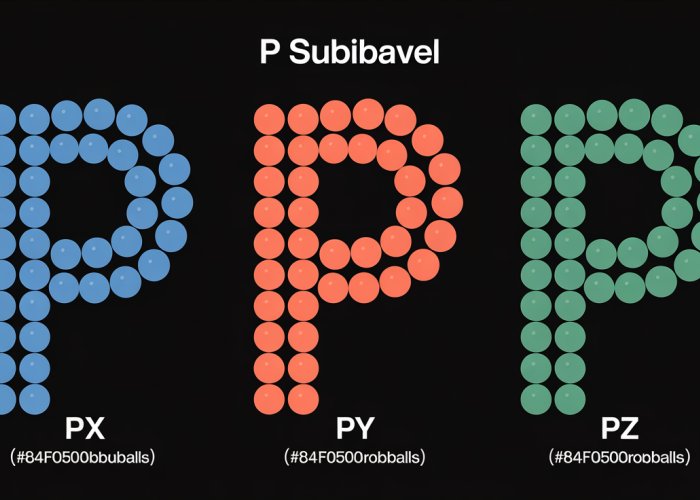
The p sublevel comprises three individual orbitals, each oriented perpendicularly to the others in three-dimensional space. These orbitals are designated as px, py, and pz, reflecting their alignment along the x, y, and z axes, respectively.
Each p orbital has a dumbbell shape, with two lobes of electron density on opposite sides of the nucleus. The nucleus itself lies at the node, a point of zero electron density, between the two lobes.
Imagine three dumbbells intersecting at their centers, each pointing along a different axis. This mental image provides a helpful representation of the spatial arrangement of the three p orbitals. Visual aids and diagrams can further enhance this understanding.
Maximum Electron Capacity: The Pauli Exclusion Principle in Action
One of the most crucial aspects of the p sublevel is its electron capacity. The p sublevel can hold a maximum of six electrons. This capacity is directly derived from the fact that the p sublevel contains three distinct p orbitals.
The Pauli Exclusion Principle
Each orbital, regardless of its shape or sublevel, can accommodate a maximum of two electrons. This limitation arises from the Pauli Exclusion Principle, a cornerstone of quantum mechanics.
The Pauli Exclusion Principle dictates that no two electrons in an atom can have the same set of four quantum numbers. These quantum numbers describe an electron’s energy level, shape of its orbital, spatial orientation, and spin.
Since each p orbital represents a unique spatial orientation (px, py, or pz), it can hold two electrons with opposite spins. Therefore, with three p orbitals, the p sublevel can accommodate a total of six electrons (3 orbitals x 2 electrons/orbital = 6 electrons).
Hund’s Rule and Orbital Filling
When filling the p orbitals with electrons, the process follows Hund’s Rule. This rule states that electrons will individually occupy each orbital within a sublevel before any orbital is doubly occupied.
Furthermore, these singly occupied orbitals will have electrons with the same spin (either all spin-up or all spin-down).
This behavior stems from the fact that electrons, being negatively charged, repel each other. By occupying separate orbitals, electrons minimize their mutual repulsion, leading to a more stable and lower-energy configuration.
Once all three p orbitals contain one electron each, only then will electrons begin to pair up in the orbitals, with each pair having opposite spins to satisfy the Pauli Exclusion Principle. Understanding Hund’s Rule is essential for accurately predicting the electron configuration of elements with partially filled p sublevels.
The Significance of P Sublevel Electrons in Chemical Behavior
Having explored the structure and capacity of the p sublevel, it’s time to appreciate its pivotal role in shaping the chemical behavior of elements. The arrangement of electrons within the p sublevel directly influences an element’s reactivity, bonding preferences, and overall chemical personality. By examining specific examples, we can see how this quantum mechanical concept translates into tangible chemical properties.
Impact on Chemical Properties and Reactivity
The electron configuration of an element, particularly the number of electrons in its outermost shell (valence electrons), dictates how readily it will interact with other atoms.
For elements with electrons occupying the p sublevel, these p electrons are often the valence electrons and thus primarily responsible for chemical bonding.
Elements strive to achieve a stable electron configuration, typically resembling that of a noble gas (octet rule).
This drive for stability motivates atoms to gain, lose, or share electrons through chemical bonds.
The number of electrons needed to complete the octet, directly related to the p sublevel occupancy, determines the type and number of bonds an element is likely to form.
For example, an element with five electrons in its p sublevel, like nitrogen, readily accepts three additional electrons to complete its octet, explaining its tendency to form three covalent bonds.
P Sublevel Electrons in Action: Case Studies
To illustrate the influence of p sublevel electrons, let’s examine a few key elements: oxygen, nitrogen, and fluorine. These elements, crucial for life and industrial processes, owe their unique chemical characteristics to their p electron configurations.
Oxygen: The Essential Oxidizer
Oxygen (O) has the electron configuration 1s² 2s² 2p⁴.
Its four p electrons in the second energy level mean it needs two more to complete its octet.
This electron deficiency makes oxygen highly electronegative and a strong oxidizing agent.
It readily forms bonds with other elements, often by accepting two electrons, to achieve a stable configuration.
This explains oxygen’s prevalence in oxides and its vital role in combustion and respiration.
Nitrogen: Versatile Bonding
Nitrogen (N) boasts the electron configuration 1s² 2s² 2p³.
With three p electrons short of a full octet, nitrogen commonly forms three covalent bonds.
It can bond with itself to form the incredibly stable diatomic molecule N₂, or with hydrogen to form ammonia (NH₃).
Nitrogen’s ability to form multiple bonds also makes it a key component of many organic molecules, including amino acids and DNA.
Fluorine: The Highly Reactive Halogen
Fluorine (F) possesses the electron configuration 1s² 2s² 2p⁵.
Its five p electrons leave it just one electron short of a full octet, making it the most electronegative element.
Fluorine aggressively attracts electrons from other atoms, forming strong bonds and exhibiting high reactivity.
It’s used in various applications, from creating non-stick coatings (Teflon) to producing pharmaceuticals.
P Sublevel Electrons: Frequently Asked Questions
Here are some common questions regarding electrons in the p sublevel, designed to clarify the main points of the guide.
What exactly is a p sublevel?
A p sublevel is a region within an electron shell of an atom. It’s characterized by a specific shape and energy level, holding electrons with similar properties. Each energy level beyond the first principal energy level (n=1) contains a p sublevel.
How many orbitals are in a p sublevel?
A p sublevel contains three orbitals. Each orbital can hold a maximum of two electrons. These orbitals are spatially oriented along the x, y, and z axes, giving them distinct directional properties.
How many electrons can the p sublevel hold?
Since there are three orbitals in a p sublevel, and each orbital can hold two electrons, the maximum number of electrons in the p sublevel is six. This is a fundamental concept for understanding electron configuration.
What happens when a p sublevel is filled with electrons?
When a p sublevel is filled with its maximum number of electrons (six), the atom gains a degree of stability. Elements with filled p sublevels, like noble gases, are generally unreactive due to this stable electron configuration.
So, now you know all about the maximum number of electrons in the p sublevel! Hopefully, this clears things up and gives you a solid foundation. Keep exploring and happy learning!