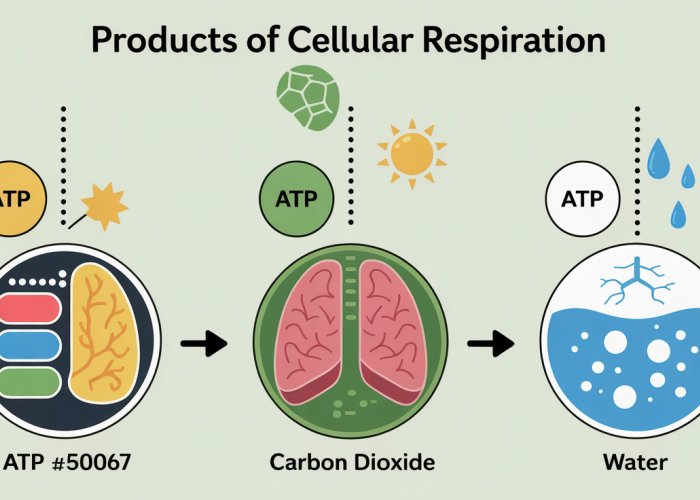
The mighty mitochondria, often hailed as the powerhouse of the cell, are central to understanding cellular energy dynamics. Glycolysis, a crucial initial step, feeds into the intricate process that yields the products of cellular respiration. Understanding this process, which is fundamental to the work of researchers at the National Institutes of Health (NIH), highlights how these products of cellular respiration fuel virtually all life processes, from a simple muscle contraction to complex thought. The significance of these products of cellular respiration can be visualized and analysed effectively by using advanced metabolic flux analysis.
Life, in all its dazzling complexity, is fundamentally an energy game. From the smallest bacterium to the largest whale, every living organism requires a constant supply of energy to fuel its existence. This energy, however, isn’t readily available in a usable form. Instead, it’s locked within the chemical bonds of the food we consume.
The process of unlocking this energy and converting it into a form that cells can use is called cellular respiration. This intricate biochemical pathway is the engine that drives life, extracting energy from food molecules like glucose and transforming it into Adenosine Triphosphate (ATP), the cell’s primary energy currency.
Cellular respiration is not a single step, but a series of interconnected reactions. These reactions not only yield ATP, but also produce a variety of byproducts, some of which are essential for other biological processes, while others are waste products that must be eliminated.
Defining Cellular Respiration
At its core, cellular respiration is the metabolic process that converts chemical energy into cellular energy. It’s a controlled oxidation of organic compounds, primarily glucose, to release energy stored in their bonds. This energy is then captured and stored in the form of ATP.
Think of it like a carefully orchestrated combustion process, but instead of fire and uncontrolled heat, it’s a series of enzyme-catalyzed reactions that efficiently extract energy.
Cellular respiration is vital for virtually all living organisms, from single-celled organisms to complex multicellular beings. It provides the energy needed for growth, movement, maintenance, and reproduction.
Food, Fuel, and Cellular Work
The food we eat is not just a source of building blocks; it’s also the fuel that powers our cells. Carbohydrates, fats, and proteins are broken down into smaller molecules, which then enter the pathways of cellular respiration.
Glucose, a simple sugar derived from carbohydrates, is a particularly important fuel. Through glycolysis, the Krebs cycle, and the electron transport chain, the energy stored within glucose is gradually released and converted into ATP.
This ATP then fuels a myriad of cellular activities, including:
-
Muscle contraction: Allowing us to move and interact with the world.
-
Active transport: Pumping molecules across cell membranes.
-
Protein synthesis: Building the proteins that carry out essential functions.
-
Nerve impulse transmission: Enabling communication within the body.
Without a constant supply of ATP, these processes would grind to a halt, and life as we know it would be impossible.
Unveiling the Products of Cellular Respiration
This article will delve into the fascinating world of cellular respiration, focusing specifically on its key products. We will explore not only ATP, but also the other important molecules generated during this process, including carbon dioxide, water, NADH, FADH2, and pyruvate.
Understanding the roles of these products is essential for comprehending how cells function, how energy flows through ecosystems, and how various diseases and health conditions can disrupt cellular metabolism. By examining these molecular players, we can gain a deeper appreciation for the elegance and efficiency of this fundamental life process.
Life, in all its dazzling complexity, is fundamentally an energy game. From the smallest bacterium to the largest whale, every living organism requires a constant supply of energy to fuel its existence. This energy, however, isn’t readily available in a usable form. Instead, it’s locked within the chemical bonds of the food we consume.
The process of unlocking this energy and converting it into a form that cells can use is called cellular respiration. This intricate biochemical pathway is the engine that drives life, extracting energy from food molecules like glucose and transforming it into Adenosine Triphosphate (ATP), the cell’s primary energy currency.
Cellular respiration is not a single step, but a series of interconnected reactions. These reactions not only yield ATP, but also produce a variety of byproducts, some of which are essential for other biological processes, while others are waste products that must be eliminated.
Defining Cellular Respiration
At its core, cellular respiration is the metabolic process that converts chemical energy into cellular energy. It’s a controlled oxidation of organic compounds, primarily glucose, to release energy stored in their bonds. This energy is then captured and stored in the form of ATP.
Think of it like a carefully orchestrated combustion process, but instead of fire and uncontrolled heat, it’s a series of enzyme-catalyzed reactions that efficiently extract energy.
Cellular respiration is vital for virtually all living organisms, from single-celled organisms to complex multicellular beings. It provides the energy needed for growth, movement, maintenance, and reproduction.
Food, Fuel, and…To fully appreciate the intricate dance of cellular respiration, we must first understand the central player in this energy game: Adenosine Triphosphate, or ATP. This remarkable molecule serves as the universal energy currency of the cell, powering virtually every process that keeps us alive and functioning.
ATP: The Cellular Energy Currency
ATP (Adenosine Triphosphate) stands as the cell’s most readily usable form of energy. It’s the molecular unit of currency, fueling a vast array of cellular processes. Without ATP, life as we know it would grind to a halt.
What is ATP? Decoding the Structure
ATP is a complex organic molecule composed of three main components:
- Adenine: A nitrogenous base, also found in DNA and RNA.
- Ribose: A five-carbon sugar.
- Triphosphate Group: A chain of three phosphate groups. This is where the energy is stored.
The key to ATP’s energy-storing capability lies in the bonds between these phosphate groups. These bonds are high-energy bonds, meaning they require a significant amount of energy to form and release a considerable amount of energy when broken.
Energy Storage and Release: Breaking the Bonds
ATP stores energy in the chemical bonds connecting its phosphate groups. When a cell needs energy to perform work, the outermost phosphate group is cleaved off through a process called hydrolysis.
This hydrolysis reaction releases energy and converts ATP into Adenosine Diphosphate (ADP) plus an inorganic phosphate group (Pi). The energy released from this bond breaking is harnessed to power cellular activities.
Think of ATP like a fully charged battery. When you use the battery, it discharges (loses a phosphate), becoming ADP. The ADP can then be "recharged" (have a phosphate added back on) to become ATP again, ready to power the next cellular process.
ATP in Action: Powering Cellular Processes
ATP powers countless cellular activities, including:
-
Muscle Contraction: The movement of muscles relies on ATP to power the interaction between actin and myosin filaments. Without ATP, muscles would remain locked in a contracted state (rigor mortis after death is a dramatic example).
-
Active Transport: Moving molecules across cell membranes against their concentration gradients requires energy, which is supplied by ATP. This is essential for maintaining proper cellular environments.
-
Protein Synthesis: Building proteins from amino acids is an energy-intensive process, and ATP provides the necessary energy.
-
Nerve Impulse Transmission: Maintaining the electrochemical gradients necessary for nerve impulse transmission requires ATP-powered ion pumps.
-
DNA and RNA Synthesis: The creation of new DNA and RNA molecules, essential for cell division and protein production, requires ATP.
These are just a few examples; ATP’s influence permeates nearly every aspect of cellular life.
ATP Synthase: The ATP Generating Machine
The continuous use of ATP necessitates a mechanism for its regeneration. This is where ATP synthase, a remarkable molecular machine, comes into play.
ATP synthase is an enzyme complex located in the inner mitochondrial membrane of eukaryotic cells (and the plasma membrane of bacteria). It uses the energy from a proton gradient to add a phosphate group to ADP, regenerating ATP.
The proton gradient is established by the electron transport chain, which pumps protons across the inner mitochondrial membrane, creating a high concentration of protons in the intermembrane space. These protons then flow back down their concentration gradient through ATP synthase, driving the rotation of a molecular rotor.
This rotation provides the energy needed to bind ADP and inorganic phosphate together, forming ATP. ATP synthase is a highly efficient and elegant example of how cells harness energy to sustain life.
In essence, ATP is the indispensable energy currency that fuels life’s processes. Its unique structure and the intricate mechanisms surrounding its synthesis and utilization highlight the remarkable elegance and efficiency of cellular biology. Understanding ATP is fundamental to comprehending the very nature of life itself.
Life, in all its dazzling complexity, is fundamentally an energy game. From the smallest bacterium to the largest whale, every living organism requires a constant supply of energy to fuel its existence. This energy, however, isn’t readily available in a usable form. Instead, it’s locked within the chemical bonds of the food we consume.
The process of unlocking this energy and converting it into a form that cells can use is called cellular respiration. This intricate biochemical pathway is the engine that drives life, extracting energy from food molecules like glucose and transforming it into Adenosine Triphosphate (ATP), the cell’s primary energy currency.
Cellular respiration is not a single step, but a series of interconnected reactions. These reactions not only yield ATP, but also produce a variety of byproducts, some of which are essential for other biological processes, while others are waste products that must be eliminated.
Defining Cellular Respiration
At its core, cellular respiration is the metabolic process that converts chemical energy into cellular energy. It’s a controlled oxidation of organic compounds, primarily glucose, to release energy stored in their bonds. This energy is then captured and stored in the form of ATP.
Think of it like a carefully orchestrated combustion process, but instead of fire and uncontrolled heat, it’s a series of enzyme-catalyzed reactions that efficiently extract energy.
Cellular respiration is vital for virtually all living organisms, from single-celled organisms to complex multicellular beings. It provides the energy needed for growth, movement, maintenance, and reproduction.
Food, Fuel, and ATP – the cell’s energy currency – are central to the story of cellular respiration. But what happens to the leftovers? What becomes of the atoms that are not incorporated into ATP? The answer lies in understanding the fate of carbon dioxide, a crucial byproduct with implications that extend far beyond the cellular level.
Carbon Dioxide: Waste Product, Environmental Factor
Carbon dioxide (CO2) often gets a bad rap, associated with pollution and climate change. But it’s crucial to remember that CO2 is also a natural and unavoidable product of cellular respiration, a process fundamental to life. It is the exhaled breath of life itself.
Let’s explore its formation, journey, and impact, examining how this seemingly simple molecule connects our cells to the global ecosystem.
The Origin of CO2: The Krebs Cycle
The story of carbon dioxide begins within the mitochondria, the powerhouses of our cells. Specifically, CO2 is generated during the Krebs cycle (also known as the citric acid cycle).
This cyclical series of chemical reactions completes the oxidation of molecules derived from glucose, releasing energy and, importantly, carbon dioxide. Each turn of the cycle releases two molecules of CO2.
Think of the Krebs cycle as a molecular dismantling process. Carbon atoms, originally part of the glucose molecule, are cleaved off and combined with oxygen to form CO2. This process extracts the remaining energy from the original fuel source.
From Cell to Lungs: The CO2 Journey
Once formed within the mitochondria, carbon dioxide embarks on a journey out of the cell.
CO2 diffuses across the cell membrane, moving from an area of high concentration (inside the cell) to an area of low concentration (the bloodstream).
Because CO2 is relatively soluble in blood, it can be transported in a few ways: dissolved directly in the plasma, bound to hemoglobin (though not at the same site as oxygen), or converted into bicarbonate ions.
However, the end destination is always the same: the lungs. Through the intricate network of blood vessels, CO2 is carried to the lungs, where it diffuses from the blood into the alveoli. Finally, it is exhaled, completing its journey out of the body.
CO2’s Role in the Carbon Cycle and Climate Change
While carbon dioxide is a natural byproduct of life, its increasing concentration in the atmosphere is a serious environmental concern. CO2 is a greenhouse gas, meaning it traps heat in the Earth’s atmosphere.
The carbon cycle describes the natural flow of carbon between the atmosphere, oceans, land, and living organisms. Cellular respiration is a key part of this cycle, releasing carbon back into the atmosphere.
However, human activities, primarily the burning of fossil fuels (coal, oil, and natural gas), have dramatically increased the amount of CO2 released into the atmosphere, overwhelming the natural carbon cycle.
This excess CO2 intensifies the greenhouse effect, leading to global warming and climate change. Understanding the source and fate of CO2, starting with its production in our own cells, is crucial for addressing this global challenge.
Therefore, while CO2 is essential in the natural processes in our bodies, it is a crucial byproduct that is very important to be monitored to prevent climate change.
Food, fuel, and a complex series of chemical reactions – it’s quite a picture we’ve painted of cellular respiration thus far. We’ve explored how cells extract energy from the food we eat, converting it into ATP, the energy currency of life. But the process isn’t solely about energy production. It also yields byproducts, each with its unique role and significance.
Water: A Vital Byproduct of Cellular Respiration
Beyond ATP, one of the crucial byproducts of this intricate process is water (H2O). Often overlooked, water plays a pivotal role in maintaining cellular health and facilitating the very reactions that sustain life. It is far more than just a waste product.
The Formation of Water in the Electron Transport Chain
The majority of water produced during cellular respiration arises from the final stage: the electron transport chain (ETC). This stage is where the real energy payoff occurs.
Electrons, carried by NADH and FADH2, are passed down a chain of protein complexes. This electron transfer drives the pumping of protons (H+) across the inner mitochondrial membrane, creating an electrochemical gradient.
At the end of the chain, these electrons must be accepted by a final electron acceptor. That acceptor is oxygen. Oxygen’s high electronegativity makes it perfectly suited to accept these electrons.
Oxygen: The Ultimate Electron Acceptor
Oxygen’s role as the final electron acceptor is paramount. It accepts the electrons and combines with hydrogen ions (protons) to form water (H2O).
This seemingly simple reaction is essential for two reasons:
- It allows the electron transport chain to continue functioning. Without a final electron acceptor to clear the way, the chain would grind to a halt.
- It maintains the electrochemical gradient that drives ATP synthase, the enzyme responsible for producing the bulk of ATP.
Without oxygen, cellular respiration becomes far less efficient, and the body must resort to less-efficient processes like fermentation.
Water’s Multifaceted Roles in the Cell
The water produced during cellular respiration isn’t simply discarded. It contributes significantly to various cellular functions:
- Cellular Hydration: Water maintains the overall hydration of the cell, providing the necessary medium for biochemical reactions to occur.
- Osmotic Balance: Water helps regulate the concentration of solutes inside and outside the cell, ensuring proper osmotic balance and preventing cells from either swelling or shrinking.
- Solvent for Biochemical Reactions: Water acts as a solvent, dissolving essential molecules and facilitating their interaction during metabolic processes. Many biochemical reactions require an aqueous environment to proceed efficiently.
- Transport: Many critical nutrients, waste products, and other molecules rely on water’s solvency to be effectively transported both in and out of the cell.
In essence, the water generated during cellular respiration is recycled and utilized for a multitude of essential functions, highlighting its importance in maintaining cellular integrity and functionality. It’s a critical component of the cellular ecosystem.
Food, fuel, and a complex series of chemical reactions – it’s quite a picture we’ve painted of cellular respiration thus far. We’ve explored how cells extract energy from the food we eat, converting it into ATP, the energy currency of life. But the process isn’t solely about energy production. It also yields byproducts, each with its unique role and significance. Now, let’s turn our attention to two crucial molecules that act as energy shuttles within the cell: NADH and FADH2.
NADH and FADH2: Electron Carriers and Energy Shuttle
NADH and FADH2 are not merely byproducts of cellular respiration; they are vital intermediaries that capture and transport the energy released during the breakdown of glucose. They act as electron carriers, bridging the gap between the earlier stages of cellular respiration (glycolysis and the Krebs cycle) and the final, ATP-generating stage: the electron transport chain.
The Genesis of NADH and FADH2
These molecules are born in the energy-extracting environments of glycolysis and the Krebs cycle.
During glycolysis, which occurs in the cytoplasm, glucose is broken down, yielding a small amount of ATP and, crucially, NADH. This process involves the transfer of electrons and hydrogen ions to NAD+ (nicotinamide adenine dinucleotide), converting it into its reduced form, NADH.
The Krebs cycle, taking place within the mitochondria, further oxidizes molecules derived from pyruvate (initially produced from glycolysis). This cycle is a hub of electron extraction, generating substantial amounts of both NADH and FADH2 (flavin adenine dinucleotide).
FADH2 is formed when FAD accepts electrons and hydrogen ions during specific oxidation reactions within the cycle.
The Electron Transport Chain: NADH and FADH2’s Delivery Service
NADH and FADH2’s primary function is to transport the high-energy electrons they’ve acquired to the electron transport chain (ETC), located in the inner mitochondrial membrane.
Think of them as delivery trucks, ferrying precious cargo to the power plant.
As NADH and FADH2 arrive at the ETC, they release their electrons.
These electrons then embark on a journey through a series of protein complexes embedded in the membrane.
Powering the Proton Pump and ATP Synthesis
The beauty of the electron transport chain lies in how it harnesses the energy released during electron transfer. As electrons move through the protein complexes, protons (H+) are actively pumped across the inner mitochondrial membrane, from the matrix to the intermembrane space.
This pumping action creates an electrochemical gradient, a difference in both charge and concentration of protons across the membrane.
This gradient stores potential energy, much like water held behind a dam.
The enzyme ATP synthase acts as a channel that allows protons to flow back down the concentration gradient, from the intermembrane space into the matrix. This flow of protons drives the rotation of a part of ATP synthase, which then catalyzes the synthesis of ATP from ADP and inorganic phosphate.
NADH contributes more to the proton gradient, leading to the production of more ATP compared to FADH2.
The Cyclical Nature of NAD+ and FAD
The story doesn’t end with NADH and FADH2 delivering their electrons. Once they’ve relinquished their cargo, they are converted back to their oxidized forms: NAD+ and FAD.
These oxidized forms are then free to participate in glycolysis and the Krebs cycle once again, accepting more electrons and continuing the cycle of energy transfer.
This cyclical nature is crucial for sustaining cellular respiration and ensuring a continuous supply of ATP. The regeneration of NAD+ and FAD allows glycolysis and the Krebs cycle to continue functioning, extracting energy from glucose and feeding the electron transport chain.
Food, fuel, and a complex series of chemical reactions – it’s quite a picture we’ve painted of cellular respiration thus far. We’ve explored how cells extract energy from the food we eat, converting it into ATP, the energy currency of life. But the process isn’t solely about energy production. It also yields byproducts, each with its unique role and significance. Now, let’s turn our attention to two crucial molecules that act as energy shuttles within the cell: NADH and FADH2. After these electron carriers deliver their precious cargo, what happens next? The answer lies, in part, with a pivotal molecule known as pyruvate.
Pyruvate: A Crossroads Molecule
Pyruvate stands as a crucial intermediary, a metabolic crossroads born from the breakdown of glucose during glycolysis. Think of it as the fork in the road for cellular respiration, where the cell must decide which path to take based on the availability of oxygen. This seemingly simple molecule holds immense power, dictating whether energy production proceeds efficiently via aerobic respiration or takes a less productive, anaerobic route.
The End Product of Glycolysis
Glycolysis, the initial stage of cellular respiration, occurs in the cytoplasm and breaks down glucose into two molecules of pyruvate. This process yields a small amount of ATP and NADH, but its primary importance lies in generating pyruvate itself.
Pyruvate, with its three-carbon structure, carries the potential energy that was once locked within the glucose molecule.
It is now ready to be further processed to extract even more energy.
Oxygen: The Deciding Factor
The fate of pyruvate hinges on a single, yet critical, factor: the presence or absence of oxygen. When oxygen is abundant, pyruvate embarks on the aerobic pathway, leading to significantly greater ATP production. However, when oxygen is scarce or absent, the anaerobic pathway, known as fermentation, takes over.
This metabolic flexibility allows cells to continue generating energy, albeit at a reduced rate, even in oxygen-deprived environments.
The Aerobic Pathway: Journey into the Mitochondria
In the presence of oxygen, pyruvate is transported into the mitochondria, the powerhouse of the cell.
Specifically, this journey takes pyruvate across both the outer and inner mitochondrial membranes.
Once inside the mitochondrial matrix, pyruvate undergoes a crucial transformation.
It is converted into Acetyl-CoA (acetyl coenzyme A), a molecule that can then enter the Krebs cycle (also known as the citric acid cycle).
This conversion is a crucial step, linking glycolysis to the subsequent stages of aerobic respiration.
Acetyl-CoA acts as the fuel that drives the Krebs cycle.
Anaerobic Pathways: Fermentation
When oxygen is limited, cells resort to fermentation, an anaerobic process that allows glycolysis to continue. Without sufficient oxygen, the electron transport chain grinds to a halt, and NADH accumulates.
Fermentation regenerates NAD+, allowing glycolysis to proceed and produce a small amount of ATP.
There are two main types of fermentation:
Lactic Acid Fermentation
In lactic acid fermentation, pyruvate is converted into lactic acid. This process occurs in muscle cells during intense exercise when oxygen supply cannot keep pace with energy demand.
The accumulation of lactic acid contributes to muscle fatigue.
Ethanol Fermentation
In ethanol fermentation, pyruvate is converted into ethanol and carbon dioxide. This type of fermentation is commonly used by yeast and bacteria in the production of alcoholic beverages and bread.
Food, fuel, and a complex series of chemical reactions – it’s quite a picture we’ve painted of cellular respiration thus far. We’ve explored how cells extract energy from the food we eat, converting it into ATP, the energy currency of life. But the process isn’t solely about energy production. It also yields byproducts, each with its unique role and significance. Now, let’s turn our attention to two crucial molecules that act as energy shuttles within the cell: NADH and FADH2. After these electron carriers deliver their precious cargo, what happens next? The answer lies, in part, with a pivotal molecule known as pyruvate.
Pyruvate stands as a crucial intermediary, a metabolic crossroads born from the breakdown of glucose during glycolysis. Think of it as the fork in the road for cellular respiration, where the cell must decide which path to take based on the availability of oxygen. This seemingly simple molecule holds immense power, dictating whether energy production proceeds efficiently via aerobic respiration or takes a less productive, anaerobic route.
Glycolysis, the initial stage of cellular respiration, occurs in the cytoplasm and breaks down glucose into two molecules of pyruvate. This process yields a small amount of ATP and NADH, but its primary importance lies in generating pyruvate itself.
Pyruvate, with its three-carbon structure, carries the potential energy that was once locked within the glucose molecule.
It is now ready to be further processed to extract even more energy.
Oxygen: The final arbiter, but before we can explore pyruvate’s ultimate fate, we must backtrack to the very beginning of energy extraction. Prepare to delve into the foundational pathway that sets the stage for all that follows: Glycolysis.
Glycolysis: The First Step in Energy Extraction
Glycolysis, derived from the Greek words for "sweet" (glykys) and "splitting" (lysis), is the initial stage of cellular respiration. It’s where the magic of energy extraction begins. This ancient and universal metabolic pathway serves as the gateway for harnessing the energy stored within glucose.
But what exactly happens during glycolysis, and why is it so vital?
The Breakdown of Glucose: A Step-by-Step Process
At its core, glycolysis is the breakdown of glucose (a six-carbon sugar) into two molecules of pyruvate (a three-carbon molecule).
This intricate process doesn’t happen in one fell swoop. It involves a series of ten enzyme-catalyzed reactions, each carefully orchestrated to modify the glucose molecule, ultimately cleaving it into two pyruvate molecules.
Think of it as a carefully choreographed dance, with each enzyme playing a specific role in transforming glucose.
Location, Location, Location: The Cytoplasmic Setting
Unlike later stages of cellular respiration that occur within the mitochondria, glycolysis takes place in the cytoplasm, the gel-like substance that fills the cell. This seemingly simple location is significant because it allows glycolysis to occur in virtually all living cells.
From bacteria to human cells, glycolysis proceeds independently of organelles, showcasing its fundamental role in life.
Energy Yield: A Small but Crucial Harvest
While glycolysis is the first step in cellular respiration, it doesn’t produce a massive amount of ATP. The net yield of ATP from glycolysis is only two molecules per glucose molecule.
In addition to ATP, glycolysis also generates two molecules of NADH (nicotinamide adenine dinucleotide), an important electron carrier that will play a crucial role in the later stages of cellular respiration.
Although the ATP yield might seem modest, it is a crucial starting point, providing the initial burst of energy needed to kickstart the entire process. More importantly, it generates the pyruvate that will fuel subsequent energy-generating pathways.
A Universal Pathway: Found in All Organisms
One of the most remarkable aspects of glycolysis is its universality. This pathway is found in virtually every organism on Earth, from the simplest bacteria to the most complex multicellular creatures.
This widespread presence suggests that glycolysis is an ancient metabolic pathway, likely predating the evolution of mitochondria. Its fundamental role in energy production has been conserved throughout the history of life.
Food, fuel, and a complex series of chemical reactions – it’s quite a picture we’ve painted of cellular respiration thus far. We’ve explored how cells extract energy from the food we eat, converting it into ATP, the energy currency of life. But the process isn’t solely about energy production. It also yields byproducts, each with its unique role and significance. Now, let’s turn our attention to two crucial molecules that act as energy shuttles within the cell: NADH and FADH2. After these electron carriers deliver their precious cargo, what happens next? The answer lies, in part, with a pivotal molecule known as pyruvate.
Pyruvate stands as a crucial intermediary, a metabolic crossroads born from the breakdown of glucose during glycolysis. Think of it as the fork in the road for cellular respiration, where the cell must decide which path to take based on the availability of oxygen. This seemingly simple molecule holds immense power, dictating whether energy production proceeds efficiently via aerobic respiration or takes a less productive, anaerobic route.
Glycolysis, the initial stage of cellular respiration, occurs in the cytoplasm and breaks down glucose into two molecules of pyruvate. This process yields a small amount of ATP and NADH, but its primary importance lies in generating pyruvate itself.
Pyruvate, with its three-carbon structure, carries the potential energy that was once locked within the glucose molecule.
It is now ready to be further processed to extract even more energy.
Oxygen: The final arbiter, but before we can explore pyruvate’s ultimate role, we must understand the next critical phase: the Krebs cycle.
Krebs Cycle (Citric Acid Cycle): Completing the Oxidation
The Krebs cycle, also known as the citric acid cycle, represents a pivotal stage in cellular respiration, acting as a metabolic furnace that further oxidizes molecules derived from pyruvate.
This cyclical pathway extracts additional energy and crucial building blocks from the initial products of glucose metabolism.
It’s within the intricate steps of the Krebs cycle that the full potential of the original glucose molecule begins to be realized.
Acetyl-CoA: The Fuel for the Cycle
The Krebs cycle doesn’t directly accept pyruvate.
Instead, pyruvate undergoes a preparatory step called oxidative decarboxylation, where it’s converted into Acetyl-CoA.
This conversion releases a molecule of carbon dioxide and links the two-carbon acetyl group to coenzyme A.
Acetyl-CoA then serves as the primary fuel that enters the Krebs cycle, initiating a series of reactions.
Location: The Mitochondrial Matrix
The Krebs cycle takes place within the mitochondrial matrix, the innermost compartment of the mitochondria.
This strategic location is essential because it positions the cycle close to the electron transport chain, the final stage of aerobic respiration, which is also located in the inner mitochondrial membrane.
The proximity allows for efficient transfer of electron carriers, NADH and FADH2, generated during the Krebs cycle, directly to the electron transport chain.
The Cyclical Nature Explained
The Krebs cycle is a true cycle, meaning that the final molecule produced in the series of reactions is also the starting molecule for the next turn of the cycle.
This cycle begins when Acetyl-CoA combines with oxaloacetate, a four-carbon molecule, forming citrate (hence the name "citric acid cycle").
Through a series of enzymatic reactions, citrate is then gradually converted back into oxaloacetate, releasing energy and regenerating the starting molecule to keep the cycle running.
Each turn of the cycle produces ATP, NADH, FADH2, and releases carbon dioxide as a byproduct.
Products of the Krebs Cycle: Energy and Building Blocks
The Krebs cycle plays a critical role in producing reduced electron carriers, NADH and FADH2.
These molecules are essential for the electron transport chain, where their electrons are used to generate a large amount of ATP through oxidative phosphorylation.
In addition to generating energy carriers, the Krebs cycle also produces carbon dioxide, which is eventually exhaled from the body.
The cycle also yields precursors for various biosynthetic pathways, contributing to the synthesis of amino acids, fatty acids, and other essential molecules.
These precursor molecules can be diverted from the cycle to create other essential molecules.
Food, fuel, and a complex series of chemical reactions – it’s quite a picture we’ve painted of cellular respiration thus far. We’ve explored how cells extract energy from the food we eat, converting it into ATP, the energy currency of life. But the process isn’t solely about energy production. It also yields byproducts, each with its unique role and significance. Now, let’s turn our attention to two crucial molecules that act as energy shuttles within the cell: NADH and FADH2. After these electron carriers deliver their precious cargo, what happens next? The answer lies, in part, with a pivotal molecule known as pyruvate.
Pyruvate stands as a crucial intermediary, a metabolic crossroads born from the breakdown of glucose during glycolysis. Think of it as the fork in the road for cellular respiration, where the cell must decide which path to take based on the availability of oxygen. This seemingly simple molecule holds immense power, dictating whether energy production proceeds efficiently via aerobic respiration or takes a less productive, anaerobic route.
Glycolysis, the initial stage of cellular respiration, occurs in the cytoplasm and breaks down glucose into two molecules of pyruvate. This process yields a small amount of ATP and NADH, but its primary importance lies in generating pyruvate itself.
Pyruvate, with its three-carbon structure, carries the potential energy that was once locked within the glucose molecule.
It is now ready to be further processed to extract even more energy.
Oxygen: The final arbiter, but before we can explore pyruvate’s ultimate fate, it’s essential to understand how the energy ultimately gets harvested.
That brings us to the Electron Transport Chain, the final, and arguably most productive, stage of aerobic cellular respiration.
Electron Transport Chain: Harvesting the Potential Energy
The Electron Transport Chain (ETC) is where the real magic of ATP production happens.
It’s the culmination of all the preceding stages of cellular respiration, where the potential energy stored within NADH and FADH2 is finally converted into a usable form.
Think of it as the grand finale, where all the hard work of glycolysis, pyruvate oxidation, and the Krebs cycle pays off in a spectacular burst of energy.
The Electron Relay Race
The ETC is a series of protein complexes embedded in the inner mitochondrial membrane.
These complexes act as a relay team, passing electrons from one to another.
The electrons initially carried by NADH and FADH2 are transferred to these complexes.
As electrons move through the chain, they release energy.
This energy isn’t directly used to make ATP, but instead is used to pump protons (H+) across the inner mitochondrial membrane.
Building the Proton Gradient: A Dam of Potential Energy
The pumping of protons creates a concentration gradient.
A higher concentration of protons builds up in the intermembrane space (the space between the inner and outer mitochondrial membranes) compared to the mitochondrial matrix.
This difference in concentration creates an electrochemical gradient, a form of potential energy, much like water held behind a dam.
The inner mitochondrial membrane is impermeable to protons.
The only way for protons to flow back down their concentration gradient is through a special channel protein called ATP synthase.
ATP Synthase: The Turbine of the Cell
ATP synthase is a remarkable molecular machine.
It acts like a turbine, using the flow of protons down their concentration gradient to drive the synthesis of ATP.
As protons flow through ATP synthase, it rotates, catalyzing the reaction that combines ADP (adenosine diphosphate) and inorganic phosphate to form ATP.
This process is called chemiosmosis, and it’s the primary mechanism by which the ETC generates ATP.
Oxygen’s Crucial Role: The Final Electron Acceptor
Oxygen plays a critical role as the final electron acceptor in the ETC.
After electrons have passed through the chain, they need to be removed to keep the process running.
Oxygen accepts these electrons and combines with protons to form water (H2O).
If oxygen isn’t available, the ETC grinds to a halt, and ATP production plummets.
This is why we need to breathe oxygen to survive; it’s essential for powering our cells.
A Highly Efficient Process
The electron transport chain is a highly efficient process.
It can generate a significant amount of ATP from a single molecule of glucose, far more than glycolysis alone.
This efficiency is crucial for meeting the energy demands of complex organisms like ourselves.
It’s a testament to the elegant and intricate design of cellular respiration.
Food, fuel, and a complex series of chemical reactions – it’s quite a picture we’ve painted of cellular respiration thus far. We’ve explored how cells extract energy from the food we eat, converting it into ATP, the energy currency of life. But the process isn’t solely about energy production. It also yields byproducts, each with its unique role and significance. Now, let’s turn our attention to two crucial molecules that act as energy shuttles within the cell: NADH and FADH2. After these electron carriers deliver their precious cargo, what happens next? The answer lies, in part, with a pivotal molecule known as pyruvate.
Pyruvate stands as a crucial intermediary, a metabolic crossroads born from the breakdown of glucose during glycolysis. Think of it as the fork in the road for cellular respiration, where the cell must decide which path to take based on the availability of oxygen. This seemingly simple molecule holds immense power, dictating whether energy production proceeds efficiently via aerobic respiration or takes a less productive, anaerobic route. Glycolysis, the initial stage of cellular respiration, occurs in the cytoplasm and breaks down glucose into two molecules of pyruvate. This process yields a small amount of ATP and NADH, but its primary importance lies in generating pyruvate itself. Pyruvate, with its three-carbon structure, carries the potential energy that was once locked within the glucose molecule. It is now ready to be further processed to extract even more energy.
Oxygen: The final arbiter, but before we can explore pyruvate’s ultimate fate, let’s zoom out and consider the grander implications of cellular respiration. How does this microscopic process, occurring within the confines of our cells, connect to the vast tapestry of life on Earth?
Cellular Respiration and Life: Connecting the Dots
Cellular respiration isn’t an isolated event; it’s a cornerstone of life’s intricate web. Its significance extends far beyond the individual cell, resonating throughout ecosystems and influencing the very health and survival of organisms.
Understanding cellular respiration provides invaluable insight into how energy flows through the biosphere and how our own bodies function.
Cellular Respiration: The Foundation of Energy Flow
Imagine the sun as the ultimate source of energy, showering the Earth with light. Plants, through photosynthesis, capture this light energy and convert it into chemical energy in the form of sugars.
These sugars then become the fuel for cellular respiration, both in plants themselves and in the organisms that consume them.
In essence, cellular respiration unlocks the energy originally captured from sunlight, making it available to power virtually all life processes.
This transfer of energy, from sunlight to plants to animals (including humans), forms the basis of food chains and food webs, which define the structure of our ecosystems.
The glucose metabolized through cellular respiration drives the essential functions of life.
A Universal Process
From the simplest bacterium to the most complex multicellular organism, cellular respiration is a fundamental requirement for life. While some organisms may utilize slightly different variations of the process, the core principles remain the same.
Cells need energy to perform essential tasks such as growth, reproduction, movement, and maintaining internal order.
The ATP produced by cellular respiration provides this energy, making it the universal energy currency of life.
The evolutionary success of cellular respiration lies in its efficiency and adaptability, allowing life to thrive in diverse environments across the globe.
Implications for Health and Disease
Understanding cellular respiration is crucial for comprehending various biological processes, health conditions, and the interconnectedness of life.
The efficiency of cellular respiration can impact everything from athletic performance to susceptibility to disease.
For example, disruptions in cellular respiration have been implicated in a range of health issues, including:
-
Metabolic disorders: Conditions like diabetes, where the body struggles to regulate blood sugar levels, directly impact the process of cellular respiration.
-
Cancer: Cancer cells often exhibit altered metabolic pathways, relying more on glycolysis (even in the presence of oxygen) than on the more efficient oxidative phosphorylation.
-
Mitochondrial diseases: These genetic disorders directly affect the mitochondria, the site of the electron transport chain, leading to impaired ATP production and a wide range of symptoms.
By studying cellular respiration, researchers can develop new strategies for treating and preventing these diseases. Furthermore, understanding how different diets and lifestyles affect cellular respiration can help individuals optimize their health and well-being.
Frequently Asked Questions: Energy Unveiled – Products of Cellular Respiration
Here are some common questions about the products of cellular respiration and how this vital process works.
What are the main products of cellular respiration?
The primary products of cellular respiration are ATP (adenosine triphosphate), carbon dioxide (CO2), and water (H2O). ATP is the energy currency of the cell, fueling various cellular processes. Carbon dioxide is a waste product exhaled from our lungs. Water is also produced as a byproduct.
Why is ATP considered the most important product of cellular respiration?
ATP is crucial because it provides the energy required for nearly all cellular activities, including muscle contraction, nerve impulse transmission, and protein synthesis. Without ATP, cells cannot function, and life would be impossible. Cellular respiration’s main goal is to generate ATP.
Besides ATP, carbon dioxide, and water, are there any other products?
While ATP, carbon dioxide, and water are the major products of cellular respiration, heat is also released as a byproduct. This heat helps maintain body temperature in warm-blooded animals.
How do the products of cellular respiration benefit a plant?
While plants primarily produce energy through photosynthesis, they also perform cellular respiration to break down sugars and create ATP for their cellular needs. The carbon dioxide produced during plant cellular respiration is often used as an input in photosynthesis. Water, another product, is essential for various plant functions.
So, that’s the lowdown on the *products of cellular respiration*! Hopefully, you now have a clearer picture of how your cells are making energy. Go forth and conquer – fueled by the wonders of cellular respiration!