The propagation of electrical signals along neurons is fundamental to nervous system function. Myelination, a process facilitated by Schwann cells in the peripheral nervous system and oligodendrocytes in the central nervous system, dramatically increases conduction velocity. Understanding the role of membrane capacitance in signal transmission helps to clarify just how much longer do un-myelinated electrical signals take?. The question of *how much longer do un-myelinated electrical signals take?* becomes increasingly relevant when considering diseases affecting myelin such as Multiple Sclerosis.
The human nervous system, a complex and intricate network, relies on rapid communication to coordinate bodily functions, process sensory information, and enable thought. This communication occurs through nerve impulses, also known as action potentials, which are electrical signals that travel along nerve fibers, or axons.
These nerve fibers, the fundamental units of neural transmission, come in two primary varieties: myelinated and unmyelinated. While both types serve the essential function of carrying signals, they differ significantly in their structure and, crucially, in the speed at which they conduct these signals.
The existence of these two distinct types of axons begs a critical question: How much slower are signals in unmyelinated axons compared to their myelinated counterparts, and why does this difference matter for the overall functioning of the nervous system?
This question forms the core of our exploration. Before we can fully appreciate the implications of this speed disparity, it’s essential to understand the basics of nerve impulses and the concept of conduction velocity.
The Essence of Nerve Impulses
At its most basic, a nerve impulse is an electrochemical signal that travels along a neuron’s axon. These impulses are the language of the nervous system, allowing neurons to communicate with each other and with other cells throughout the body, such as muscle cells and glands. Without nerve impulses, we wouldn’t be able to move, feel, think, or even breathe.
These impulses are essential for everything we do.
Myelinated vs. Unmyelinated Axons: A Tale of Two Fibers
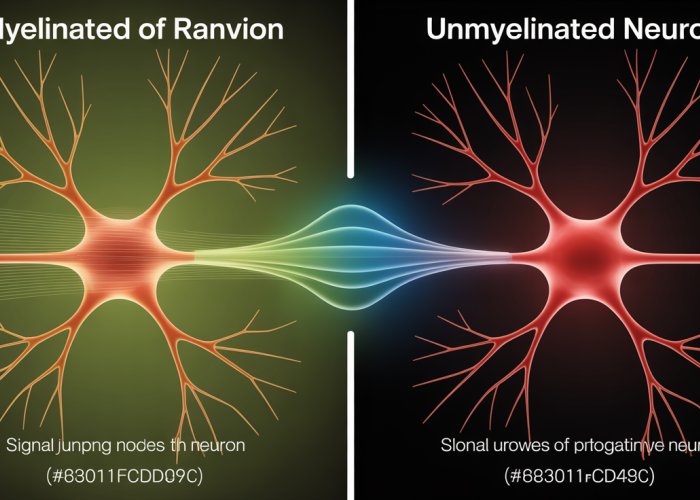
The critical distinction between myelinated and unmyelinated axons lies in the presence or absence of a myelin sheath. Myelin, a fatty substance produced by specialized glial cells, wraps around the axon, forming an insulating layer.
This insulation dramatically affects the way nerve impulses are conducted. Myelinated axons allow for saltatory conduction, where the signal "jumps" between gaps in the myelin sheath called Nodes of Ranvier, greatly accelerating transmission speed. Unmyelinated axons, lacking this insulation, conduct signals more slowly and continuously.
The Central Question: Quantifying the Speed Disparity
The difference in conduction speed between myelinated and unmyelinated axons is substantial. While myelinated axons can transmit signals at speeds ranging from 18 to 150 meters per second, unmyelinated axons typically conduct signals at a much slower rate, often around 0.5 to 2 meters per second. This disparity raises further questions about the functional roles of each axon type and the evolutionary pressures that have shaped their characteristics.
Understanding Conduction Velocity
Conduction velocity is a measure of the speed at which an action potential travels along an axon. It is a crucial parameter in neurophysiology, as it reflects the efficiency and speed of neural communication. Factors such as myelination, axon diameter, and temperature can all influence conduction velocity.
The Foundation: How Neuronal Signals are Transmitted
Having established the stage with the two primary axon types, it’s time to delve into the underlying mechanism that powers nerve impulse transmission. Understanding how these signals are generated and propagated is crucial for appreciating the differences in conduction velocity between myelinated and unmyelinated axons.
At the heart of neuronal communication lies the action potential, a transient, self-regenerating electrical signal that sweeps along the neuron’s axon, allowing neurons to relay information over considerable distances.
Defining the Action Potential
The action potential is the fundamental unit of electrical signaling in neurons.
Think of it as a rapid, short-lasting change in the electrical potential across the neuron’s plasma membrane. This change moves like a wave, traveling from one end of the neuron to the other.
At rest, the neuron maintains a negative electrical potential inside relative to the outside, known as the resting membrane potential (typically around -70 mV).
An action potential occurs when this resting potential is disrupted by a stimulus, causing a rapid depolarization (the inside becomes more positive) followed by repolarization (returning to the negative resting state).
Generation and Propagation: A Step-by-Step Explanation
The process of action potential generation and propagation can be broken down into several key steps:
-
Stimulus and Depolarization: A stimulus, whether from another neuron or a sensory receptor, causes the neuron’s membrane to depolarize.
-
Threshold: If the depolarization reaches a certain threshold (typically around -55 mV), voltage-gated sodium channels open.
-
Rapid Depolarization: The influx of positively charged sodium ions causes a rapid and dramatic depolarization of the membrane, driving the potential towards a positive value.
-
Repolarization: Shortly after sodium channels open, they inactivate and voltage-gated potassium channels open. The efflux of potassium ions then repolarizes the membrane, bringing it back towards the resting potential.
-
Hyperpolarization: The potassium channels remain open for a brief period, causing a transient hyperpolarization (the membrane potential becomes more negative than the resting potential) before returning to the resting state.
-
Refractory Period: Following an action potential, there’s a brief refractory period during which the neuron is less excitable, preventing backward propagation of the signal.
The Role of Electrical Signals (Voltage Changes)
The action potential is fundamentally an electrical phenomenon.
It’s driven by changes in the membrane potential. This is caused by movement of ions across the neuronal membrane. The opening and closing of ion channels are key.
These voltage changes are not just random fluctuations; they are carefully orchestrated by the neuron to transmit information in a reliable and directional manner.
The strength and frequency of action potentials can encode different types of information, allowing neurons to communicate complex signals.
Voltage-Gated Ion Channels: Gatekeepers of the Action Potential
Voltage-gated ion channels are transmembrane proteins that selectively allow specific ions (such as sodium or potassium) to pass through the membrane, and their opening and closing are regulated by changes in the membrane potential.
They are essential for generating and propagating action potentials. These channels act as gatekeepers, opening and closing in response to changes in voltage.
This allows for a rapid and controlled flow of ions across the membrane, driving the depolarization and repolarization phases of the action potential.
Without these channels, neurons would not be able to generate the rapid electrical signals required for communication.
Propagation: Electrical Signals Move Down the Axon
Propagation refers to how the electrical signal, the action potential, moves down the axon.
The action potential generated at one location on the axon triggers depolarization in the adjacent region, leading to the opening of voltage-gated ion channels in that region, and thus regenerating the action potential.
This process continues along the length of the axon, ensuring that the signal is transmitted without weakening. The action potential propagates in one direction, away from the cell body, due to the refractory period following the passing of the electrical signal.
The speed of propagation, or conduction velocity, is a crucial factor in determining how quickly information can be transmitted throughout the nervous system. The conduction velocity is the term that separates myelinated and unmyelinated fibers, a topic we will cover later.
Having explored the fundamental mechanisms of action potential generation and propagation, it’s time to consider the biological innovation that drastically accelerates these signals: myelination. This process, akin to installing a high-speed rail line alongside a local track, transforms the efficiency of neuronal communication.
Myelination: The Neural Superhighway
Myelination represents a pivotal evolutionary adaptation that significantly enhances the speed and efficiency of nerve impulse transmission. This process involves the ensheathment of axons by specialized glial cells, forming a myelin sheath that acts as an insulator, fundamentally altering the way electrical signals propagate along the neuron.
The Formation of the Myelin Sheath
Myelination is orchestrated by two types of glial cells: oligodendrocytes in the central nervous system (CNS) and Schwann cells in the peripheral nervous system (PNS). These cells wrap themselves concentrically around the axon, creating multiple layers of lipid-rich membranes.
Each oligodendrocyte can myelinate portions of multiple axons, whereas each Schwann cell myelinates a single segment of one axon. This wrapping process compacts the cell membrane, expelling the cytoplasm and forming a dense, insulating layer composed primarily of lipids.
The resulting myelin sheath is not continuous; instead, it is punctuated by short, unmyelinated segments called Nodes of Ranvier. These nodes are crucial for the mechanism of saltatory conduction.
Myelin as an Insulator: Blocking Current Leakage
The primary function of the myelin sheath is to act as an electrical insulator. Like the plastic coating around an electrical wire, myelin prevents the leakage of ions across the axonal membrane.
This insulation increases the membrane resistance and decreases the membrane capacitance, making it more difficult for ions to flow across the membrane in myelinated regions. As a result, the electrical signal can travel further down the axon without dissipating.
This is because myelin reduces the number of open ion channels required for propagation along the myelinated region.
Saltatory Conduction: Jumping the Gaps
The presence of Nodes of Ranvier, the gaps in the myelin sheath, is critical to the process of saltatory conduction.
In myelinated axons, action potentials do not need to be regenerated at every point along the membrane, as they do in unmyelinated axons. Instead, the action potential "jumps" from one node to the next.
At each Node of Ranvier, there is a high concentration of voltage-gated sodium channels. When the depolarizing current from the previous node reaches a node, it triggers an influx of sodium ions, regenerating the action potential.
This "jumping" greatly increases the speed of conduction because the action potential only needs to be generated at the nodes.
The current flows passively along the myelinated segments between nodes, which is much faster than the active regeneration required in unmyelinated axons. Saltatory conduction also reduces the energy expenditure of neurons, as fewer ions need to be pumped across the membrane.
A Historical Note: Hodgkin-Huxley and the Ionic Basis of Action Potentials
While the structure and function of myelin are crucial for understanding conduction velocity, the fundamental understanding of the ionic basis of the action potential itself was established by Alan Hodgkin and Andrew Huxley.
Their work, primarily conducted on the giant axons of squid, elucidated the roles of sodium and potassium ions in generating the action potential.
Hodgkin and Huxley developed a mathematical model that described how the flow of these ions through voltage-gated channels creates the characteristic depolarization and repolarization phases of the action potential.
Although their model didn’t directly address myelination, it provided the essential framework for understanding how ion channels function, which is critical for understanding both continuous and saltatory conduction. Their model earned them the Nobel Prize in Physiology or Medicine in 1963 and laid the foundation for subsequent research on neuronal signaling.
The insulation provided by myelin drastically alters the speed and efficiency of signal transmission, as we’ve seen. But what about neurons that lack this specialized wrapping? How do unmyelinated axons manage to conduct signals, and what are the trade-offs involved in this seemingly slower mode of communication?
Unmyelinated Axons: Slow and Steady Wins the Race (Sometimes)
Signal transmission in unmyelinated axons relies on a fundamentally different mechanism than saltatory conduction. Instead of "jumping" between Nodes of Ranvier, the action potential must be regenerated continuously along the entire length of the axon. This process, while reliable, is considerably slower.
Continuous Regeneration of the Action Potential
In unmyelinated fibers, the action potential propagates like a wave moving down a line. At each point along the axon, the influx of sodium ions (Na+) during the action potential depolarizes the adjacent region of the membrane. This depolarization triggers the opening of voltage-gated sodium channels in that neighboring region, initiating a new action potential.
This cycle repeats itself, with each segment of the axon actively participating in regenerating the signal.
Therefore, the action potential doesn’t simply travel passively; it is actively rebuilt at every location. This continuous regeneration ensures the signal’s strength doesn’t diminish over distance.
However, the sequential activation of ion channels across the entire axonal membrane is inherently slower than the "jumping" mechanism of saltatory conduction.
Factors Influencing Conduction Speed
Several factors influence how quickly an action potential propagates down an unmyelinated axon. The two primary determinants are membrane capacitance and membrane resistance.
Membrane Capacitance
Membrane capacitance refers to the ability of the axonal membrane to store electrical charge. A higher capacitance means that more charge must accumulate to change the membrane potential, making it slower to reach the threshold for firing an action potential.
Thus, lower membrane capacitance will promote faster conduction.
Membrane Resistance
Membrane resistance, conversely, is the resistance to ion flow across the membrane. Higher membrane resistance means less leakage of ions, allowing the depolarizing current to spread further and faster.
Therefore, higher membrane resistance leads to faster conduction velocities.
Axon Diameter
Perhaps the most significant factor influencing conduction speed in unmyelinated axons is the diameter of the axon. A larger axon diameter reduces the internal resistance to current flow, allowing for faster and more efficient propagation of the action potential.
Think of it like water flowing through a pipe: a wider pipe offers less resistance and allows for a greater flow rate. Similarly, a larger axon provides a lower resistance path for the electrical current associated with the action potential.
Myelinated vs. Unmyelinated: A Speed Comparison
The difference in conduction speed between myelinated and unmyelinated axons is substantial. Myelinated axons can conduct signals at speeds ranging from 3 to 120 meters per second (m/s), while unmyelinated axons typically conduct signals at speeds of 0.5 to 2 m/s.
This means that a signal traveling along a myelinated axon can reach its destination up to 60 times faster than a signal traveling along an unmyelinated axon!
The disparity in speed highlights the significant advantage conferred by myelination in terms of rapid communication within the nervous system. However, the slower conduction in unmyelinated axons is not without its purpose, as we’ll explore in the next section.
The Evolutionary Advantage: Why Unmyelinated Axons Still Exist
It’s tempting to view myelination as a purely superior evolutionary adaptation, a neural upgrade that renders unmyelinated fibers obsolete. Yet, the persistence of unmyelinated axons throughout the nervous system reveals a more nuanced story. Why haven’t these "slower" fibers been entirely replaced by their faster, myelinated counterparts? The answer lies in a delicate balance of trade-offs, where speed isn’t the only factor determining evolutionary success.
Energy Efficiency: A Key Consideration
Myelination, while boosting conduction velocity, comes at a metabolic cost. The production and maintenance of myelin sheaths are energy-intensive processes. Oligodendrocytes (in the central nervous system) and Schwann cells (in the peripheral nervous system) require a constant supply of energy to synthesize and maintain these insulating layers.
Unmyelinated axons, by contrast, have significantly lower energy demands. The continuous regeneration of the action potential requires less metabolic investment per unit length than maintaining a myelin sheath.
In situations where high-speed transmission is not critical, the energy savings offered by unmyelinated axons can be a significant advantage, particularly in systems operating continuously or under metabolic constraints.
Flexibility and Plasticity: The Adaptive Edge
The rigid structure imposed by myelin, while facilitating rapid signal propagation, can also limit axonal flexibility. Unmyelinated axons, lacking this constraint, exhibit greater plasticity and adaptability. This flexibility allows for more dynamic remodeling of neural circuits in response to changing demands or experiences.
This characteristic is particularly important in developmental processes and learning, where neural connections are constantly being refined. The ability to easily modify synaptic connections and axonal pathways is crucial for adapting to new environments and acquiring new skills.
Space Efficiency: Packing More into Less
In certain regions of the nervous system, space is at a premium. The compact nature of unmyelinated axons allows for a higher density of neurons within a given volume. This is particularly important in densely packed brain regions where computational power depends on a large number of interconnected neurons.
For example, the olfactory bulb, responsible for processing smell, relies heavily on unmyelinated axons to maximize the number of sensory neurons that can be accommodated. Similarly, in certain areas of the cerebral cortex, the increased density afforded by unmyelinated fibers facilitates complex information processing.
Prevalence in Specific Systems
The functional advantages of unmyelinated axons explain their prominence in specific systems. Pain fibers, for instance, often rely on unmyelinated C fibers. While the slower conduction speed might seem disadvantageous, it ensures that pain signals are processed with a degree of deliberation, rather than triggering instantaneous, reflexive responses.
The autonomic nervous system, which regulates involuntary functions like heart rate and digestion, also utilizes unmyelinated axons extensively. Here, the emphasis is on sustained, energy-efficient control rather than rapid, reflexive action. The slower conduction speeds are sufficient for maintaining homeostasis and coordinating long-term physiological processes.
Implications for Neuroscience
Understanding the distinct roles and advantages of both myelinated and unmyelinated axons is crucial for a comprehensive understanding of the nervous system. It highlights the evolutionary pressures that have shaped neural architecture and provides insights into the functional organization of different brain regions.
Furthermore, it is critical to consider these differences when studying neurological disorders. Damage to myelin, as seen in multiple sclerosis, has drastically different effects than damage to unmyelinated axons. Understanding which fiber types are affected and how their function is compromised is key to developing effective treatments and therapies. The ongoing exploration of these neural structures promises deeper insights into the complexities of the nervous system.
Conduction Velocity: Measuring the Speed of Neural Signals
The preceding sections have illuminated the contrasting mechanisms of signal propagation in myelinated and unmyelinated axons, revealing the trade-offs between speed, energy efficiency, and flexibility. But how do we quantify this "speed" and why is it such a crucial factor in understanding nerve function? Conduction velocity provides a precise metric for assessing the functionality of neural circuits and their ability to rapidly transmit information throughout the nervous system.
Defining Conduction Velocity
Conduction velocity is the speed at which an action potential propagates along an axon. It’s typically measured in meters per second (m/s) and represents the distance traveled by the signal per unit of time. This measurement is not merely an academic exercise; it’s a vital indicator of the health and efficiency of nerve fibers.
A healthy nervous system relies on rapid and precise communication between neurons. Conduction velocity directly reflects this communication efficiency.
Measuring Conduction Velocity: A Multifaceted Approach
Determining conduction velocity involves stimulating a nerve and recording the time it takes for the action potential to reach a recording electrode at a known distance away.
This can be achieved through various techniques, including:
- Electrophysiology: This involves direct electrical stimulation of the nerve and recording the resulting electrical activity.
- Nerve Conduction Studies (NCS): A common diagnostic test used in clinical settings to assess nerve damage by measuring the speed of electrical impulses along a nerve.
- Computational Modeling: Using mathematical models to simulate action potential propagation and estimate conduction velocity based on axon properties.
The choice of method depends on the specific research question or clinical need. Each method offering varying degrees of precision and applicability.
The Myelin Factor: Supercharging Conduction Velocity
As previously discussed, myelination dramatically increases conduction velocity. This is due to saltatory conduction, where the action potential "jumps" between Nodes of Ranvier. This reduces the need for continuous regeneration of the action potential along the entire axon.
In myelinated axons, conduction velocities can range from 20 m/s to over 120 m/s. A stark contrast compared to the 0.5 m/s to 10 m/s typically observed in unmyelinated axons.
The presence and integrity of myelin are therefore primary determinants of signal transmission speed.
Other Factors Influencing Conduction Velocity
While myelination is the most significant factor, several other parameters also influence conduction velocity:
Axon Diameter
Larger axon diameters generally lead to faster conduction velocities. This is because larger axons have lower internal resistance. Lower internal resistance allows for more efficient current flow. Think of it like water flowing through a wide pipe versus a narrow one.
Temperature
Temperature also plays a role, with higher temperatures generally increasing conduction velocity. This is because temperature affects the kinetics of ion channels involved in action potential generation. However, extreme temperatures can impair nerve function.
Axon Health
The overall health and integrity of the axon is also critical. Damage or disease affecting the axon structure or ion channel function can significantly reduce conduction velocity.
Conduction Velocity: A Window into Neurophysiology
Conduction velocity is a critical parameter in neurophysiology. It allows us to assess the functional integrity of nerve fibers and neural circuits.
Changes in conduction velocity can indicate various neurological disorders, including:
- Multiple Sclerosis (MS): Demyelination slows down conduction velocity.
- Peripheral Neuropathies: Damage to peripheral nerves reduces conduction velocity.
- Guillain-Barré Syndrome: An autoimmune disorder affecting myelin and slowing down conduction velocity.
By measuring conduction velocity, clinicians and researchers can gain valuable insights into the underlying pathophysiology of these conditions. Conduction velocity helps monitor disease progression and evaluate the effectiveness of treatments.
FAQs: Understanding Unmyelinated Nerve Signals
Here are some frequently asked questions about the speed of unmyelinated nerve signals.
What makes unmyelinated nerve signals slower?
Unmyelinated nerve signals travel by a continuous process of depolarization along the entire axon. This requires every section of the membrane to undergo changes, taking more time than jumping between nodes of Ranvier in myelinated axons. This is how much longer do un-myelinated electrical signals take compared to myelinated.
Why do some nerves remain unmyelinated?
While speed is a benefit, myelination also requires space and energy. Certain nerve pathways, like those involved in pain sensation and autonomic functions, may prioritize resource efficiency or fine-grained control over rapid transmission. This allows subtle, localized responses.
Are there any benefits to slower, unmyelinated signals?
Yes, unmyelinated fibers can be more energy-efficient and less prone to certain types of damage. Their continuous conduction can also provide a more stable and reliable signal in some circumstances, because the signal is not being "jumped" between segments.
How much slower are unmyelinated signals compared to myelinated ones?
Myelinated nerve signals can travel up to 100 meters per second, while unmyelinated signals typically travel at less than 1 meter per second. So, as you can imagine how much longer do un-myelinated electrical signals take, the myelinated pathways are significantly faster—often by a factor of 100 or more.
So, next time you’re pondering the intricacies of the nervous system, remember how myelination impacts signal speed and consider just how much longer do un-myelinated electrical signals take? Thanks for diving in!