The Bohr model, a cornerstone of atomic physics, provides a simplified yet insightful framework for understanding electron behavior. Cobalt, a transition metal with diverse industrial applications, presents a complex electronic structure ripe for Bohr model analysis. Specifically, the energy levels of cobalt electrons, as predicted by the Bohr model, can be investigated using spectroscopic techniques prevalent at institutions like the National Institute of Standards and Technology (NIST). The ‘bohr model for cobalt’, while not perfectly accurate due to its limitations in describing multi-electron atoms, can still offer a valuable starting point for understanding its chemical properties and interactions with tools like X-ray photoelectron spectroscopy (XPS).
Unlocking Cobalt’s Secrets: Bohr Model Explained!
Understanding the behavior of elements like Cobalt begins with understanding its atomic structure. The Bohr model, while simplified, provides a valuable foundational insight. This explanation focuses on applying the Bohr model specifically to Cobalt, addressing its limitations and significance in visualizing atomic structure.
Introduction to the Bohr Model
The Bohr model, proposed by Niels Bohr in 1913, describes the atom as a nucleus surrounded by electrons orbiting in specific, quantized energy levels or shells. These shells are often denoted by the principal quantum number, n, which can be any positive integer (n=1, 2, 3, etc.).
Key Principles of the Bohr Model
- Quantized Energy Levels: Electrons can only exist in specific orbits around the nucleus. Each orbit corresponds to a discrete energy level.
- Electron Transitions: Electrons can jump between energy levels by absorbing or emitting energy in the form of photons. The energy of the photon is equal to the difference in energy between the two levels.
- Defined Orbits: Electrons move around the nucleus in defined circular paths, similar to planets orbiting the sun.
Applying the Bohr Model to Cobalt
Cobalt (Co) has an atomic number of 27, meaning it has 27 protons in its nucleus and, in a neutral atom, 27 electrons orbiting the nucleus. Distributing these 27 electrons according to the Bohr model is a key step in understanding Cobalt’s chemical behavior.
Electron Configuration of Cobalt in the Bohr Model
The Bohr model uses a 2n2 rule to determine the maximum number of electrons that can occupy each shell.
- n=1 (K shell): Can hold up to 2(1)2 = 2 electrons.
- n=2 (L shell): Can hold up to 2(2)2 = 8 electrons.
- n=3 (M shell): Can hold up to 2(3)2 = 18 electrons.
- n=4 (N shell): Can hold up to 2(4)2 = 32 electrons, although in Cobalt’s case, we won’t need all 32.
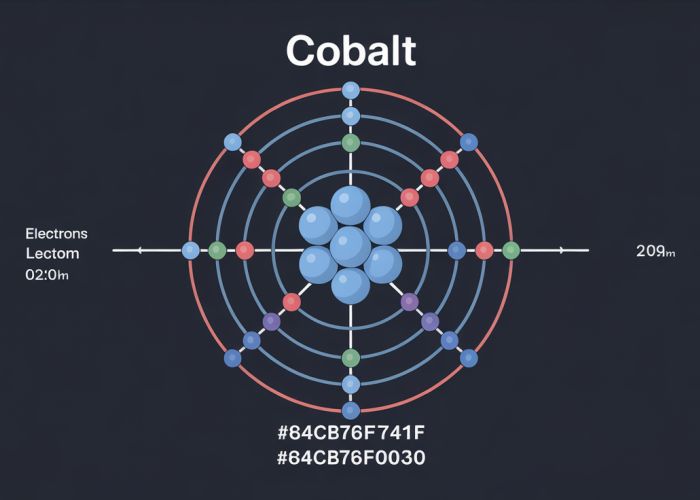
Therefore, the electron configuration of Cobalt using the Bohr model can be represented as: 2-8-17. This means that Cobalt has:
- 2 electrons in the innermost shell (K shell)
- 8 electrons in the second shell (L shell)
- 17 electrons in the third shell (M shell)
It’s important to note that this configuration, derived from the Bohr model, is a simplification. While it helps visualize the general arrangement, the more accurate electron configuration of Cobalt is 1s2 2s2 2p6 3s2 3p6 4s2 3d7. The Bohr model doesn’t fully account for the complexities introduced by subshells (s, p, d, f) and electron-electron interactions.
Bohr Model Diagram of Cobalt
A diagram illustrating the Bohr model for Cobalt would show a central nucleus labeled "Cobalt (Co) +27". Surrounding this nucleus would be three concentric circles representing the K, L, and M shells. The K shell would have two electrons depicted, the L shell would have eight, and the M shell would have seventeen.
Limitations of the Bohr Model
While useful for introductory concepts, the Bohr model has significant limitations, especially when applied to elements beyond hydrogen.
- Fails for Multi-Electron Atoms: The Bohr model struggles to accurately predict the properties of atoms with more than one electron. It doesn’t account for electron-electron repulsion.
- Doesn’t Explain Spectral Line Intensities: The model doesn’t explain why some spectral lines are brighter than others.
- Oversimplified Orbits: Electrons do not orbit the nucleus in fixed, circular paths. They exist in three-dimensional orbitals described by quantum mechanics.
- Violation of Heisenberg’s Uncertainty Principle: The Bohr model suggests that we can know both the position and momentum of an electron simultaneously, which violates Heisenberg’s Uncertainty Principle.
Why Still Use the Bohr Model?
Despite its limitations, the Bohr model is valuable because:
- Simple Visualization: It provides a simple, easily understandable visual representation of atomic structure and energy levels.
- Foundation for Quantum Mechanics: It introduced the concept of quantized energy levels, which is fundamental to quantum mechanics.
- Pedagogical Tool: It serves as a useful stepping stone for understanding more complex atomic models.
Advanced Atomic Models and Cobalt
More accurate descriptions of Cobalt’s atomic structure rely on quantum mechanics, including the Schrödinger equation and the concept of atomic orbitals. These models provide a more complete picture of electron distribution and behavior, accounting for electron spin, orbital shapes, and electron-electron interactions. Understanding the limitations of the Bohr model is crucial for appreciating the necessity and accuracy of these advanced models.
Frequently Asked Questions: Cobalt and the Bohr Model
Got questions about how the Bohr model applies to Cobalt? Here are some common questions answered to help solidify your understanding.
Why is the Bohr model for Cobalt important to understand?
The Bohr model, while simplified, gives us a basic understanding of Cobalt’s electronic structure. Specifically, it illustrates how Cobalt’s electrons are arranged in energy levels or shells around the nucleus. This helps explain its chemical behavior.
How many electron shells does the Bohr model show for Cobalt?
The Bohr model for Cobalt (Co) shows four electron shells. The innermost shell holds a maximum of two electrons, the next holds eight, the third can hold up to 18, and the fourth houses the remaining electrons.
Is the Bohr model for Cobalt a completely accurate representation?
No, the Bohr model is a simplified depiction. It’s useful for visualizing electron arrangements but doesn’t account for electron orbitals’ complex shapes and interactions. The quantum mechanical model offers a more accurate picture.
What information does the Bohr model for Cobalt provide about its reactivity?
While not fully comprehensive, the Bohr model hints at Cobalt’s reactivity. Specifically, the number of electrons in its outermost shell dictates how it will interact with other elements to form chemical bonds.
Alright, hope that deep dive into the bohr model for cobalt made things a little clearer! Keep exploring, and you’ll be mastering atomic structure in no time. Until next time!



